Abstract
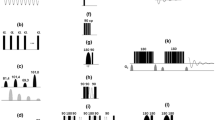
Recently we have shown that HMQC spectra of protonated methyl groups in high molecular weight, highly deuterated proteins have large enhancements in sensitivity and resolution relative to HSQC-generated data sets. These enhancements derive from a TROSY effect in which complete cancellation of intra-methyl 1H-1H and 1H-13C dipolar interactions occurs for 50% of the signal in the case of HMQC, so long as the methyl is attached to a molecule tumbling in the macromolecular limit (Tugarinov, V., Hwang, P.M., Ollerenshaw, J.E., Kay, L.E. J. Am. Chem. Soc. (2003) 125, 10420–10428; Ollerenshaw, J.E., Tugarinov, V. and Kay, L.E. Magn. Reson. Chem. (2003) 41, 843–852. The first demonstration of this effect was made for isoleucine δ1 methyl groups in a highly deuterated 82 kDa protein, malate synthase G. As with 1H-15N TROSY spectroscopy high levels of deuteration are critical for maximizing the TROSY effect. Here we show that excellent quality methyl TROSY spectra can be recorded on U-[2H] Ileδ1-[13CH3] Leu,Val-[13CH3/12CD3] protein samples, significantly extending the number of probes available for structural and dynamic studies of high molecular weight systems.
Similar content being viewed by others
References
Bax, A., Griffey, R.H. and Hawkings, B.L. (1983) J. Magn. Reson., 55, 301–315.
Bodenhausen, G. and Rubin, D.J. (1980) Chem. Phys. Lett., 69, 185–189.
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR, 6, 277–293.
Eletsky, A., Moreira, O., Kovacs, H. and Pervushin, K. (2003) J. Biomol. NMR, 26, 167–179.
Gardner, K.H. and Kay, L.E. (1997) J. Am. Chem. Soc., 119, 7599–7600.
Gardner, K.H. and Kay, L.E. (1998) Annu. Rev. Biophys. Biomol. Struct., 27, 357–406.
Gardner, K.H., Rosen, M.K. and Kay, L.E. (1997) Biochemistry, 36, 1389–1401.
Goto, N.K. and Kay, L.E. (2000) Curr. Opin. Struct. Biol., 10, 585–592.
Goto, N.K., Gardner, K.H., Mueller, G.A., Willis, R.C. and Kay, L.E. (1999) J. Biomol. NMR, 13, 369–374.
Gross, J.D., Gelev, V.M. and Wagner, G. (2003) J. Biomol. NMR, 235–242.
Hajduk, P.J., Augeri, D.J., Mack, J., Mendoza, R., Yang, J.G., Betz, S.F. and Fesik, S.W. (2000) J. Am. Chem. Soc., 122, 7898–7904.
Howard, B.R., Endrizzi, J.A. and Remington, S.J. (2000) Biochemistry, 39, 3156–3168.
Janin, J., Miller, S. and Chothia, C. (1988) J. Mol. Biol., 204, 155–164.
Kay, L.E. and Prestegard, J.H. (1987) J. Am. Chem. Soc., 3829–3835.
Kay, L.E. and Torchia, D.A. (1991) J. Magn. Reson., 95, 536–547.
Metzler, W.J., Wittekind, M., Goldfarb, V., Mueller, L. and Farmer, B.T. (1996) J. Am. Chem. Soc., 118, 6800–6801.
Mueller, G.A., Choy, W.Y., Yang, D., Forman-Kay, J.D., Venters, R.A. and Kay, L.E. (2000) J. Mol. Biol., 300, 197–212.
Mueller, L. (1979) J. Am. Chem. Soc., 101, 4481–4484.
Mueller, N., Bodenhausen, G. and Ernst, R.R. (1987) J. Magn. Reson., 75, 297–334.
Nicholson, L.K., Kay, L.E., Baldisseri, D.M., Arango, J., Young, P.E., Bax, A. and Torchia, D.A. (1992) Biochemistry, 31, 5253–5263.
Ollerenshaw, J.E., Tugarinov, V. and Kay, L.E. (2003) J. Magn. Reson. Chem., 41, 843–852.
Pervushin, K., Riek, R., Wider, G. and Wüthrich, K. (1997) Proc. Natl. Acad. Sci. USA, 94, 12366–12371.
Pervushin, K., Riek, R., Wider, G. and Wüthrich, K. (1998) J. Am. Chem. Soc., 120, 6394–6400.
Pintacuda, G. and Otting, G. (2002) J. Am. Chem. Soc., 124, 372–373.
Salzmann, M., Pervushin, K., Wider, G., Senn, H. and Wüthrich, K. (2000) J. Am. Chem. Soc., 122, 7543–7548.
Skrynnikov, N.R., Mulder, F.A.A., Hon, B., Dahlquist, F.W. and Kay, L.E. (2001) J. Am. Chem. Soc., 123, 4556–4566.
Tugarinov, V. and Kay, L.E. (2003) J. Mol. Biol., 327, 1121–1133.
Tugarinov, V., Hwang, P.M., Ollerenshaw, J.E. and Kay, L.E. (2003) J. Am. Chem. Soc., 125, 10420–10428.
Tugarinov, V., Muhandiram, R., Ayed, A. and Kay, L.E. (2002) J. Am. Chem. Soc., 124, 10025–10035.
Werbelow, L.G. and Marshall, A.G. (1973) J. Magn. Reson., 299–313.
Wider, G. and Wüthrich, K. (1999) Curr. Opin. Struct. Biol., 9, 594–601.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tugarinov, V., Kay, L.E. An Isotope Labeling Strategy for Methyl TROSY Spectroscopy. J Biomol NMR 28, 165–172 (2004). https://doi.org/10.1023/B:JNMR.0000013824.93994.1f
Issue Date:
DOI: https://doi.org/10.1023/B:JNMR.0000013824.93994.1f