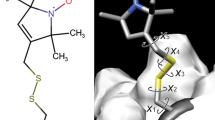
Abstract
The 46-kD enzyme 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase catalyzes the condensation of shikimate-3-phosphate (S3P) and phosphoenolpyruvate to form EPSP. The reaction is inhibited by N-(phosphonomethyl)-glycine (Glp), which, in the presence of S3P, binds to EPSP synthase to form a stable ternary complex. We have used solid-state NMR and molecular modeling to characterize the EPSP synthase–S3P–Glp ternary complex. Modeling began with the crystal coordinates of the unliganded protein, published distance restraints, and information from the chemical modification and mutagenesis literature on EPSP synthase. New inter-ligand and ligand-protein distances were obtained. These measurements utilized the native 31P in S3P and Glp, biosynthetically 13C-labeled S3P, specifically 13C and 15N labeled Glp, and a variety of protein-15N labels. Several models were investigated and tested for accuracy using the results of both new and previously published rotational-echo double resonance (REDOR) NMR experiments. The REDOR model is compared with the recently published X-ray crystal structure of the ternary complex, PDB code 1G6S. There is general agreement between the REDOR model and the crystal structure with respect to the global folding of the two domains of EPSP synthase and the relative positioning of S3P and Glp in the binding pocket. However, some of the REDOR data are in disagreement with predictions based on the coordinates of 1G6S, particularly those of the five arginines lining the binding site. We attribute these discrepancies to substantive differences in sample preparation for REDOR and X-ray crystallography. We applied the REDOR restraints to the 1G6S coordinates and created a REDOR-refined xray structure that agrees with the NMR results.
Similar content being viewed by others
References
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. and Lipman, D.J. (1990) J. Mol. Biol., 215, 403–410.
Anderson, K.S. and Johnson, K.A. (1990) Chem. Rev., 90, 1131–1149.
Anderson, K.S., Sikorski, J.A. and Johnson, K.A. (1988) Biochemistry, 27, 1604–1610.
Berisio, R., Lamzin, V.S., Sica, F., Wilson, K.S., Zagari, A. and Mazzarella, L. (1999) J. Mol. Biol., 292, 845–854.
Berman, H.M., Westbrook, J., Feng, Z., Gilliand, G., Bhat, T.N., Weissig, H., Shindyalov, I.N. and Bourne, P.E. (2000) Nucl. Acids Res., 28, 235–242.
Beusen, D.D., McDowell, L.M., Slomczynska, U. and Schaefer, J. (1995) J. Med. Chem., 38, 2742–2747.
Castellino, S., Leo, G.C., Sammons, R.D. and Sikorski, J.A. (1991) J. Org. Chem., 56, 5176–5181.
Chan, J.C.C. and Eckert, H. (2000) J. Magn. Reson., 147, 170–178.
Christensen, A.M. and Schaefer, J. (1993) Biochemistry, 32, 2868–2873.
Duncan, K., Lewendon, A. and Coggins, J.R. (1984) FEBS Lett., 170, 59–63.
Franz, J.E., Mao, M.K. and Sikorski, J.A. (1997) Glyphosate: A Unique Global Herbicide, American Chemical Society, Washington, DC.
Gehring, K., Zhang, X., Hall, J., Nikaido, H. and Wemmer, D.E. (1998) Biochem. Cell Biol., 76, 189–197.
Goetz, J.M. and Schaefer, J. (1997) J. Magn. Reson., 127, 147–154.
Gullion, T. and Schaefer, J. (1989a) J. Magn. Reson., 81, 196–200.
Gullion, T. and Schaefer, J. (1989b) Adv. Magn. Reson., 13, 57–83.
Gullion, T. and Schaefer, J. (1991) J. Magn. Reson., 92, 439–442.
Gullion, T. and Vega, S. (1992) Chem. Phys. Lett., 194, 423–428.
Gullion, T., Baker, D.B. and Conradi, M.S. (1990) J. Magn. Reson., 89, 479–484.
Hing, A.W., Tjandra, N., Cottam, P.F., Schaefer, J. and Ho, C. (1994) Biochemistry, 33, 8651–8661.
Hodsdon, M.E. and Cistola, D.P. (1997) Biochemistry, 36, 2278–2290.
Huang, D.B., Ainsworth, C.F., Stevens, F.J. and Schiffer, M. (1996) Proc. Natl. Acad. Sci. U.S.A., 93, 7017–7021.
Huynh, Q.K. (1990) J. Biol. Chem., 265, 6700–6704.
Huynh, Q.K. (1991) Arch. Biochem. Biophys., 284, 407–412.
Huynh, Q.K. (1992) Biochem. Biophys. Res. Commun., 185, 317–322.
Huynh, Q.K. (1993) Biochem. J., 290, 525–530.
Huynh, Q.K., Kishore, G.M. and Bild, G.S. (1988a) J. Biol. Chem., 263, 735–739.
Huynh, Q.K., Bauer, S.C., Bild, G.S., Kishore, G.M. and Borgmeyer, J.R. (1988b) J. Biol. Chem., 263, 11636–11639.
Jacob, G.S., Schaefer, J., Garbow, J.R. and Stejskal, E.O. (1987) J. Biol. Chem., 262, 254–259.
Kim, D.H., Tucker-Kellogg, G.W., Lees, W.J. and Walsh, C.T. (1996) Biochemistry, 35, 5435–5440.
Knowles, P.F. and Sprinson, D.B. (1970) Meth. Enzymol., 17A, 351–352.
Krekel, F., Oecking, C., Amrhein, N. and Macheroux, P. (1999) Biochemistry, 38, 8864–8878.
Kurinov, I.V. and Harrison, R.W. (1995) Acta Cryst. D Biol. Cryst., D51, 98–109.
Larsen, T.M., Benning, M.M., Wesenberg, G.E., Rayment, I. and Reed, G.H. (1997) Arch. Biochem. Biophys., 345, 199–206.
Leo, G.C., Castellino, S., Sammons, R.D. and Sikorski, J.A. (1992) Bioorg. Med. Chem. Lett., 2, 151–154.
Lukin, J.A, Kontaxis, G., Simplaceanu, V., Yuan, Y., Bax, A. and Ho, C. (2003) Proc. Natl. Acad. Sci. U.S.A., 100, 517–520.
Marshall, G.R., Beusen, D.D., Kociolek, K., Redlinski, A.S., Leplawy, M.T., Pan, Y. and Schaefer, J. (1990) J. Am. Chem. Soc., 112, 963–966.
McDowell, L.M., Klug, C.A., Beusen, D.D. and Schaefer, J. (1996a) Biochemistry, 35, 5395–5403.
McDowell, L.M., Schmidt, A., Cohen, E.R., Studelska, D.R. and Schaefer, J. (1996b) J. Mol. Biol., 256, 160–171.
Mehta, A.K., Hirsh, D.J., Oyler, N., Drobny, G.P. and Schaefer, J. (2000) J. Magn. Reson., 145, 156–158.
Millican, R.C. (1970) Meth. Enzymol., 17A, 352–354.
Mousedale, D.M. and Coggins, J.R. (1995) Planta, 163, 241–249.
O'Connor, R.D. and Schaefer, J. (2002) J. Magn. Reson., 154, 46–52.
Olins, P.O., Devine, C.S., Rangwala, S.H. and Kavka, K.S. (1988) Gene, 73, 227–235.
Padgette, S.R., Re, D.B., Gasser, C.S., Eichholtz, D.A., Frazier, R.B., Hironaka, C.M., Levine, E.B., Shah, D.M., Fraley, R.T. and Kishore, G.M. (1991) J. Biol. Chem., 266, 22364–22369.
Padgette, S.R., Smith, C.E., Huynh, Q.K. and Kishore, G.M. (1988) Arch. Biochem. Biophys., 266, 254–262.
Pan, Y., Gullion, T. and Schaefer, J. (1990) J. Magn. Reson., 90, 330–340.
Quiocho, F.A. (1990) Phil. Trans. Royal Soc. London. Ser. B Biol. Sci., 326, 341–351; Discussion 351–352.
Raleigh, D.P., Levitt, M.H. and Griffin, R.G. (1988) Chem. Phys. Lett., 146, 71–76.
Renatus, M., Bode, W., Huber, R., Sturzebecher, J. and Stubbs, M.T. (1998) J. Med. Chem., 41, 5445–5456.
Rueppel, M.L., Brightwell, B.B., Schaefer, J. and Marvel, J.T. (1977) J. Agric. Food Chem., 25, 517–528.
Schönbrunn, E., Eschenburg, S., Shuttleworth, W.A., Schloss, J.V., Amrhein, N., Evans, J.N.S. and Kabsch, W. (2001) Proc. Natl. Acad. Sci. USA, 98, 1376–1380.
Selvapandiyan, A., Ahmad, S., Majumder, K., Arora, N. and Bhatnagar, R.K. (1996) Biochem. Mol. Biol. Int., 40, 603–610.
Selvapandiyan, A., Majumder, K., Fattah, F.A., Ahmad, S., Arora, N. and Bhatnagar, R.K. (1995) FEBS Lett., 374, 253–256.
Shuttleworth, W.A. and Evans, J.N.S. (1994) Biochemistry, 33, 7062–7068.
Shuttleworth, W.A. and Evans, J.N.S. (1996) Arch. Biochem. Biophys., 334, 37–42.
Shuttleworth, W.A., Pohl, M.E., Helms, G.L., Jakeman, D.L. and Evans, J.N.S. (1999) Biochemistry, 38, 296–302.
Stalker, D.M., Hiatt, W.R. and Comai, L. (1985) J. Biol. Chem., 260, 4724–4728.
Stallings, W.C., Abdel-Meguid, S.S., Lim, L.W., Shieh, H.-S., Dayringer, H.E., Leimgruber, N.K., Stegeman, R.A., Anderson, K.S., Sikorski, J.A., Padgette, S.R. and Kishore, G.M. (1991) Proc. Nat. Acad. Sci. USA, 88, 5046–5050.
Stauffer, M.E., Young, J.K. and Evans, J.N.S. (2001) Biochemistry, 40, 3951–3957.
Stauffer, M.E., Young, J.K., Helms, G.L. and Evans, J.N.S. (2001) FEBS Lett., 499, 182–186.
Steinrücken, H.C. and Amrhein, N. (1980) Biochem. Biophys. Res. Commun., 94, 1207–1212.
Stubbs, M.T., Reyda, S., Dullweber, F., Moller, M., Klebe, G., Dorsch, D., Mederski, W.W.K.R. and Wurziger, H. (2002) ChemBioChem., 3, 246–249.
Studelska, D.R., Klug, C.A., Beusen, D.D., McDowell, L.M. and Schaefer, J. (1996) J. Am. Chem. Soc., 118, 5476–5477.
Studelska, D.R., McDowell, L.M., Espe, M.P., Klug, C.A. and Schaefer, J. (1997) Biochemistry, 36, 15555–15560.
Wang, J., Balazs, Y.S. and Thompson, L.K. (1997) Biochemistry, 36, 1699–1703.
Waugh, D.S. (1996) J. Biomol. NMR, 8, 184–192.
Weiss, U. and Mingioli, E.S. (1956) J. Am. Chem. Soc., 78, 2894–2898.
Weldeghiorghis, T.K. and Schaefer, J. (2003) J. Magn. Reson., in press.
Wishart, D.S., Sykes, B.D. and Richards, F.M. (1991) J. Mol. Biol., 222, 311–333.
Wu, J., Xiao, C., Yee, A.F., Goetz, J.M. and Schaefer, J. (2000) Macromolecules, 33, 6849–6852.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McDowell, L.M., Poliks, B., Studelska, D.R. et al. Rotational-echo double-resonance NMR-restrained model of the ternary complex of 5-enolpyruvylshikimate-3-phosphate synthase. J Biomol NMR 28, 11–29 (2004). https://doi.org/10.1023/B:JNMR.0000012864.70184.48
Issue Date:
DOI: https://doi.org/10.1023/B:JNMR.0000012864.70184.48