Abstract
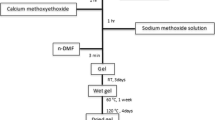
A new sol–gel synthesis route to organic free Na2O–Al2O3–P2O5 xerogels was developed. These xerogels contain P2O5 up to 67.5 mole % and Al2O3 up to 20 mole % and could be melted to homogeneous glasses in air at significantly lower temperatures than those applied using the conventionally melting method. Aluminum isopropoxide dissolved in isopropanol, orthophosphoric acid dissolved in isopropanol and sodium nitrate were used as precursors for Al2O3, P2O5 and Na2O respectively. Clear stable alcoholic solutions were prepared in the presence of HNO3. Addition of HNO3 in the concentration used, enabled homogeneous mixing of the precursors, controlled over the gelation step and prevented the fast precipitation of the AlPO4 crystalline phase which breaks down the glass formation ability. The sol–gel route developed was investigated using different analytical tools. Differential thermal analysis (DTA) coupled with thermo-gravimetric (TG) analysis indicated that neither organic residues nor sodium nitrate residues were present in the dried gels after gelation. Fourier transform infrared spectroscopy (FTIR) showed the presence of all characteristic phosphate groups and bonds in the dried gels, especially the P=O and the P–O–P bonds. Solid state 31P Magic angle spinning nuclear magnetic resonance (MAS NMR) revealed the formation of Q2 chain-like polyphosphates of variable polymerization degrees in the dried gels. Solid state 27Al MAS NMR revealed that octahedrally coordinated Al is the preferred moiety in the dried gels. However, powder X-ray diffraction (XRD) investigation indicated the presence of crystalline Q1 oligomeric pyrophosphate species in the dried gels. Isotropic chemical shifts belonging to these pyrophosphate species were also recorded in the 31P MAS NMR spectra. Characterization of the prepared glasses showed that their properties are in very good agreement with those glasses prepared by the conventionally melting method.
Similar content being viewed by others
References
M. B. VolfGlass Science and Technology, Vol. 7 (Elsevier, 1984).
H. Schmidt, J. Non-Cryst. Solids 100 (1988) 51.
H. Schmidt and H. Wolter, ibid. 121 (1990) 428.
J. Livage and C. Sanchez, ibid. 145 (1992) 11.
L. Coury, F. Babonneau, M. Henry and J. Livage, C.R. Acad. Sci. Paris 309 Ser. II (1989) 799.
S. Prabakar, K. J. Rao and C. N. R. Rao, Mater. Res. Bull. 26 (1991) 805.
M. A. Harmer and A. J. Vega, Solid State Nucl. Magn. Reson. 5 (1995) 35.
DIN 52 324, Prüfung von Glas, Bestimmung der Transformationstemperatur (1984).
N. N. Greenwood and A. EarnshawChemistry of the Elements(Pergamon Press, Oxford, 1984).
A. F. Holleman and E. WibergLehrbuch der Anorganischen Chemie(Walter de Gruyter, Berlin, 1985).
H. Suzuki, H. Saito and T. Hayashi, J. Mater. Sci. 19 (1984) 396.
B. Bridge and N. D. Patel, J. Non-Cryst. Solids 91 (1987) 27.
E. A. Hayri and M. Greenblatt, ibid. 94 (1987) 387.
E. A. Hayri, M. Greenblatt, P. Pruna and R. Gerhardt, ibid. 111 (1989) 167.
L. Sheng and J. Zarzycki, ibid. 112 (1989) 428.
M. Laczka and M. Ciecinska, J. Sol-Gel Sci. Technol. 3 (1994) 219.
L. Montagne, S. Donze and G. Palavit, Fundamentals of Glass Science and Technology, 3rd ESG Conference (1995).
R. J. Kirkpatrick and R. K. Brow, Solid State Nucl. Magn. Reson. 5 (1995) 9.
R. K. Brow, R. J. Kirkpatrick and G. L. Turner, J. Am. Ceram. Soc. 76 (1993) 919.
R. K. Brow, ibid. 76 (1993) 913.
B. E. Yoldas, Am. Ceram. Soc. Bull. 54 (1975) 286; and Am. Ceram. Soc. Bull. 54 (1975) 289.
F. Gomez, P. Vast, Ph. Llewellyn and F. Rouquerol, J. Non-Cryst. Solids 222 (1997) 415.
J. Van WazerPhosphorus and its Compounds, Vols. 1 and 2 (Wiley Interscience, NY, 1951).
N. J. Kreidl and W. A. Weyl, J. Am. Ceram. Soc. 24 (1941) 372.
D. E. Day and G. E. Rindone, ibid. 45 (1962) 489.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abo-Naf, S.M., Ghoneim, N.A. & EI-Batal, H.A. Preparation and characterization of sol–gel derived glasses in the ternary Na2O–Al2O3–P2O5 system. Journal of Materials Science: Materials in Electronics 15, 273–282 (2004). https://doi.org/10.1023/B:JMSE.0000024226.51362.de
Issue Date:
DOI: https://doi.org/10.1023/B:JMSE.0000024226.51362.de