Abstract
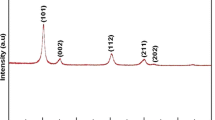
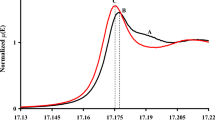
Zirconium oxide superacids with different sulphate ion contents were prepared from Zr(SO4)2 using urea as precipitating agent. These oxides were subjected to different heating temperatures. Characterization was carried out by means of infrared spectroscopy, X-ray diffraction and nitrogen adsorption studies. The surface acidity was determined by the method described by Boehm [1]. Infrared results confirmed the presence of the sulphate ions and suggested the gradual lowering through heat-treatment of the symmetry of the sulphate ions by complex formation. Surface acidity was favored when the sulphate ions were in a bidentate state of ligation. The latter ligands seemed in turn to be stabilized by water. The strongest acidity was displayed in systems where a high concentration of bidentated sulphate ions was present in conjunction with high surface areas and micropore volumes.
Similar content being viewed by others
References
H. P. Boehm, Discuss. Faraday Soc. 52 (1971) 264.
K. Yamagushi, T. Jin and K. Tanabe, Mater. Chem. Phys. 17 (1987) 3.
K. Arata, Appl. Catal. A: Gen. 146 (1996) 3.
G. K. Chuah, S. Jaenike, S. A. Cheong and K. S. Chang, ibid. 145 (1996) 267.
X. Song and A. Sayari, Chemtech. (1995) 27.
M. Waquif, J. Bachelier, O. Saur and J. C. Lavallay, J. Mol. Catal. 72 (1992) 127.
J. M. Parera, Catal. Today 15 (1992) 481.
T. Yamagushi, Appl. Catal. 61 (1990) 1.
R. Srinvasan, D. Taulbee and B. H. Davis, Catal. Lett. 9 (1991) 1.
K. Arata and M. Hino, Mater. Chem. Phys. 26 (1990) 213.
M. T. Tran, N. S. Gnep, G. Szabo and M. Guisnet, Appl. Catal. A: Gen. 171 (1998) 207.
W. Stichert and F. Schuth, J. Catal. 174 (1998) 242.
M. Hino, S. Kobayashi and K. Arata, J. Amer. Chem. Soc. 101 (1979) 6439.
V. Parvulescu, S. Coman, P. Grange and V. I. Parvulescu, Appl. Catal. A: Gen. 176 (1999) 27.
E. Platero and P. Mentruit, Mater. Lett. 14 (1992) 318.
B. H. Davis, R. A. Keogh and R. Srinivasan, Catal. Today 20 (1994) 219
C. Zhang, G. Meng, D. Han and X. Zeng, Cailiao Yanjiu Xuebao 9 (1995) 259.
E. E. Platero, M. P. Mentruit, C. O. Arean and A. Zecchina, J. Catal. 162 (1996) 268.
K. Arata, M. Hino and N. Yamagata, Bull. Chem. Soc. Jpn. 63 (1990) 244.
T. Riemer, D. Spielbaker, M. Hunger, G. A. H. Mekhemer and H. Knozinger, J. Chem. Soc. Chem. Commun. (1994) 1181.
F. Garin, L. Seyfried, P. Girard, G. Maire, A. Abdulsamad and J. Sommer, J.Catal. 151 (1995) 26.
V. Adeeva, J. W. De Haan, J. Janchen, G. D. Lei, V. Schunemann, L. J. M. Van De Ven, W. M. H. Sachtler and R. A. Van Santen, ibid. 151 (1995) 364.
W. Stichert, F. Schuth, S. Kuba and H. Knozinger, ibid. 198 (2001) 277.
C. A. Vera and J. M. Parera, ibid. 165 (1997) 254.
F. Babou, G. Coudurier and J. C. Vedrine, ibid. 152 (1995) 341.
K. Nakamoto, “Infrared and Raman Spectra of Inorganic and Coordination Compounds” (Wiley, New York, 1986).
M. L. Hair, J. Phys. Chem. 74 (1970) 1290.
J. Ragai, J. Chem. Tech. Biotech. 40 (1987) 143.
N. D. Parkyns, “Chemisorption and Catalysis” (Hepple Publications, London, 1970).
K. Nakamoto, “Infrared and Raman Spectra of Inorganic and Coordination Compounds” (Wiley, New York, 1978) p. 239.
J. Ragai and K. S. W. Sing, J. Colloid Interf. Sci. 101 (1984) 369.
S. J. Gregg and K. S. W. Sing, “Adsorption, Surface Area and Porosity” (Academic Press, London, 1982) p. 98.
O. Saur, M. Bensitel, A. B. M. Saad, J. C. Lavalley, C. P. Tripp and B. A. Morrow, J. Catal. 99 (1986) 104.
D. Tichit, D. Elalami and F. Figueras, ibid. 163 (1996) 18.
S. J. Gregg and K. S. W. Sing, “Adsorption, Surface Area and Porosity” (Academic Press, London, 1982) p. 195.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ramadan, A.R., Yacoub, N. & Ragai, J. Surface and related studies in sulphated oxides of zirconium. Journal of Materials Science 39, 1383–1388 (2004). https://doi.org/10.1023/B:JMSC.0000013901.14430.20
Issue Date:
DOI: https://doi.org/10.1023/B:JMSC.0000013901.14430.20