Abstract
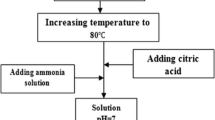
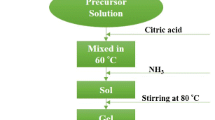
Fine barium hexaferrite (BaFe12O19) powder consisting of platelike particles with the magnetoplumbite structure, 30–60 nm in size, is synthesized at 550°C via rapid oxidation of a suspension of barium and iron(II) hydroxides with NaClO. The magnetic properties of BaFe12O19 powders prepared by modified coprecipitation and spray pyrolysis processes are described. The coercivity of fine BaFe12O19 powders is shown to be lower than that of bulk material, which is due to the presence of a magnetically inactive layer of noncollinear spins in the basal plane of the hexagonal particles. BaFe12O19 particles less than 10 nm in size are shown to be in a superparamagnetic state.
Similar content being viewed by others
REFERENCES
Pankhurst, Q.A. and Pollard, R.S., Fine-Particle Mag-netic Oxides, J. Phys.: Condens. Matter, 1993, vol. 5, pp. 8487–8508.
Shin, H.S. and Kwon, S.-J., Low Temperature Synthesis of Barium Ferrite Particles with a Modified Coprecipitation Method, Proc. VI Int. Conf. on Ferrites, Tokyo/Kyoto, 1992, pp. 1402–1405.
Barb, D., Diamandescu, L., Rusi, A., et. al., Preparation of Barium Hexaferrite by Hydrothermal Method: Structure and Magnetic Properties, J. Mater. Sci., 1986, vol. 21, pp. 1118–1122.
Gorner, P., Pfeiffer, H., Sinn, E., et. al., Nanocrystalline M-Type Hexaferrite Powders: Preparation, Magnetic Properties, IEEE Trans. Magn., 1994, vol. 30, no. 2, pp. 714–716.
Li, X., Lu, G., and Li, S., Synthesis and Properties of Strontium Ferrites Ultrafine Powders, J. Mater. Sci. Lett., 1996, vol. 15, pp. 397–399.
Pankov, V., Modified Aerosol Synthesis for Nanoscale Hexaferrite Particles Preparation, Mater. Sci. Eng., A, 1997, vol. 224, pp. 101–106.
Burukhin, A.A., Churagulov, B.R., Oleinikov, N.N., and Meskin, P.E., Synthesis of Nanocrystalline Ferrite Pow-ders in Hydrothermal and Supercritical Solutions, Zh. Neorg. Khim., 2001, vol. 46, no. 5, pp. 735–741.
Perng, L.H., Tung, I.-C., and Chin, T.S., Synthesis and Properties of Nanocrystalline d-FeOOH, J. Phys. IV, 1997, vol. 7, no. 3, pp. C1-519–C1-520.
Buyanov, R.A. and Krivoruchko, O.P., Crystallization Theory for Poorly Soluble Metal Hydroxides and Scientific Principles behind the Use of Such Substances in the Preparation of Catalysts, Kinet. Katal., 1976, vol. 17, no. 3, pp. 765–774.
Hibst, H., Magnetic Pigments for Recording Information, J. Magn. Magn. Mater., 1982, vol. 74, pp. 193–202.
Sarda, Ch., Contribution à l'étude de l'héxaferrite de baryum et des ferrites spinelles dopés au baryum, Thèse, Toulouse: Univ. de Toulouse, 1984.
Oh, J.H., Namikawa, T., and Satu, M., Wet Processes for the Preparation of Ba-Ferrite Powders, J. Jpn. Chem. Soc., 1978, p. 636.
Henis, A. and Pankov, V.V., The Modified Aerosol Pyrolysis as a Preparation of Hexaferrite Particles for Magnetic Media, J. Phys. IV, 1997, vol. 7, no. 3, pp. C1-74--C1-748.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pankov, V.V. Synthesis of BaFe12O19 Powder by Modified Coprecipitation and Spray Pyrolysis Methods. Inorganic Materials 40, 979–984 (2004). https://doi.org/10.1023/B:INMA.0000041333.79255.1f
Issue Date:
DOI: https://doi.org/10.1023/B:INMA.0000041333.79255.1f