Abstract
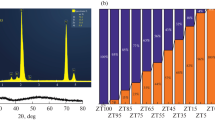
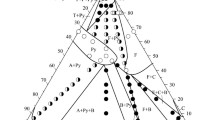
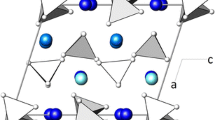
The phases crystallizing in the Na2CO3–ZrO2(cr)–SiO2(silica gel)–H2O system at 500°C, 0.1 GPa, ZrO2 : SiO2 = 1 : 2 to 1 : 6, and ZrO2 : Na2CO3 = 1 : 1 to 1 : 3 are ZrSiO4 (zircon), Na2ZrSi4O11 (vlasovite), Na2ZrSi2O7 (parakeldyshite), and Na4Zr2Si3O12 (NASICON). The structures of the three Na–Zr silicates are built up of the same polyhedral structural units—M octahedra and T tetrahedra—connected so as to form a three-dimensional MT framework. The crystallization behavior of these phases can be understood in terms of the matrix assembly of crystal structures from suprapolyhedral structural units in the form of cyclic clusters. The structures of Na2ZrSi4O11 and Na2ZrSi2O7 contain invariant six-polyhedron precursors of composition Na2 M 2 T 4, each made up of two ZrO6 octahedra and four SiO4 tetrahedra. The formation of Na4Zr2Si3O12 involves four-polyhedron Na-containing clusters of composition Na2 M 2 T 2.
Similar content being viewed by others
REFERENCES
Inorganic Crystal Structure Database (ICSD), Karlsruhe: Gmelin-Inst. für Anorganische Chemie & FIC, 2002.
Powder Diffraction File, Newtown Square: Int. Center for Diffraction Data.
Ilyushin, G.D. and Blatov, B.A., Crystal Chemistry of Zirconosilicates and Their Analogs: Topological Classi-fication of MT-Frameworks and Suprapolyhedral Invariants, Acta Crystallogr., Sect. B: Struct. Sci., 2002, vol. 58, no. 2, pp. 198–218.
Ilyushin, G.D., Phase Relations in the Na<Subscript>2</Subsrcipt>CO<Subscript>3</Subsrcipt>-ZrO<Subscript>2</Subscript>-SiO<Subscript>2</Subscript>-H<Subscript>2</Subscript>O System at 0.1 and 0.05 GPa and 450°C, Neorg. Mater., 2002, vol. 38, no. 12, pp. 1470–1478 [Inorg. Mater. (Engl. Transl.), vol. 38, no. 12, pp. 1249?1257].
Ilyushin, G.D., Hydrothermal Crystallization in the System NaOH-ZrO<Subscript>2</Subscript>-SiO<Subscript>2</Subscript>-H<Subscript>2</Subscript>O at 450°C: Na4Zr2Si5O16 H<Subscript>2</Subscript>O, Na<Subscript>8</Subscript>ZrSi<Subscript>6</Subscript>O<Subscript>18</Subscript>, Na<Subscript>3</Subscript>HZrSi<Subscript>2</Subscript>O<Subscript>8</Subscript>, and Na<Subscript>4</Subscript>Zr<Subscript>2</Subscript>Si<Subscript>3</Subscript>O<Subscript>12</Subscript> Phase Relations, Zh. Neorg. Khim., 2003, vol. 48, no. 6, pp. 1002–1111.
Ilyushin, G.D., Dem'yanets, L.N., Ilyukhin, V.V., and Belov, N.V., Formation of Mineral Analogs and Synthetic Phases in the Hydrothermal System NaOH-ZrO<Subscript>2</Subscript>-SiO<Subscript>2</Subscript>-H<Subscript>2</Subscript>O, Dokl. Akad. Nauk SSSR, 1983, vol. 271, no. 5, pp. 1133–1136.
Bortun, A.I., Bortun, L.N., and Clearfield, A., Hydrothermal Synthesis of Sodium Zirconium Silicates and Characterization of Their Properties, Chem. Mater., 1997, vol. 9, no. 8, pp. 1854–1864.
Jale, S.R., Ojo, A., and Fitch, F.R., Synthesis of Microporous Zirconosilicates Containing ZrO<Subscript>6</Subscript> Octahedra and SiO<Subscript>4</Subscript> Tetrahedra, Chem. Commun., 1999, no. 5, pp. 411–412.
Ferreira, P., Ferreira, A., Rocha, J., and Sousa, M.R., Synthesis and Structural Characterization of Zirconium Silicates, Chem. Mater., 2001, vol. 13, no. 2, pp. 355–363.
Poojary, D.M., Bortun, A.I., Bortun, L.N., and Clearfield, A., Syntheses and X-ray Powder Structures of K<Subscript>2</Subscript>ZrSi<Subscript>3</Subscript>O<Subscript>9</Subscript> H<Subscript>2</Subscript>O and Its Ion-Exchanged Phases with Na and Cs, Inorg. Chem., 1997, vol. 36, no. 14, pp. 3072?3079.
Anthony, K., Cheetham, A.K., Ferey, G., and Loiseau, T., Open-Framework Inorganic Materials, Angew. Chem., 1999, vol. 38, no. 22, pp. 3268–3292.
Korzhinskaya, V.S. and Nekrasov, I.Ya., Stability of ZrO<Subscript>2</Subscript> + SiO<Subscript>2</Subscript> + ZrSiO<Subscript>4</Subscript> Associates in Carbonate Melts at 200-400°C and 1 kbar, Dokl. Akad. Nauk, 1996, vol. 347, no. 3, pp. 383–386.
Korzhinskaya, V.S. and Nekrasov, I.Ya., Stability of ZrO<Subscript>2</Subscript> + SiO<Subscript>2</Subscript> + ZrSiO<Subscript>4</Subscript> Associates in Alkaline Melts at 500°C and 1 kbar, Dokl. Akad. Nauk, 1998, vol. 359, no. 2, pp. 205–208.
Ilyushin, G.D. and Dem'yanets, L.N., Germanaty chetyrekhvalentnykh metallov (Germanates of Tetravalent Metals), Moscow: VINITI, 1989.
Ilyushin, G.D. and Dem'yanets, L.N., A Model for the Matrix Assembly of Crystal Structures, in Fizika kristallizatsii (Physics of Crystallization), Moscow: Nauka, 2002, pp. 82–169.
Ilyushin, G.D., Modelirovanie protsessov samoorganizatsii v kristalloobrazuyushchikh sistemakh (Modeling of Self-organization Processes in Crystal-Forming Systems), Moscow: URSS, 2003.
Ilyushin, G.D. and Dem'yanets, L.N., Crystal Chemistry of the Framework K-Zr Silicates K<Subscript>2</Subscript>ZrSi<Subscript>6</Subscript>O<Subscript>15</Subscript>, K<Subscript>2</Subscript>ZrSi<Subscript>3</Subscript>O<Subscript>9</Subscript>, and K2ZrSi<Subscript>2</Subscript>O<Subscript>7</Subscript>: Combinatorial-Topological Analysis and Structural Modeling, Zh. Neorg. Khim., 2002, vol. 47, no. 9, pp. 1480–1489.
Ilyushin, G.D., Hydrothermal Crystallization in the System NaOH-TiO2-SiO<Subscript>2</Subscript>-H<Subscript>2</Subscript>O at 500°C and 0.1 GPa: Na<Subscript>2</Subsrcipt>TiSi<Subscript>4</Subscript>O<Subscript>11</Subscript>, Na<Subscript>2</Subsrcipt>Ti<Subscript>2</Subscript>Si<Subscript>2</Subscript>O<Subscript>9</Subscript>, and Na<Subscript>2</Subsrcipt>TiSiO<Subscript>5</Subscript> Phase Relations, Zh. Neorg. Khim., 2004, vol. 49, no. 8.
Ilyushin, G.D., Blatov, B.A., and Zakutkin, Yu.A., Crystal Chemistry of Orthosilicates and Their Analogs: Classification by Topological Types of Suprapolyhedral Structural Units, Acta Crystallogr., Sect. B: Struct. Sci., 2002, vol. 58, no. 6, p. 948–964.
Ilyushin, G.D., Hydrothermal Crystallization in the Systems CsOH-ZrO<Subscript>2</Subscript>-SiO<Subscript>2</Subscript>-H<Subscript>2</Subscript>O and CsCl-ZrO<Subscript>2</Subscript>-SiO<Subscript>2</Subscript>-H<Subscript>2</Subscript>O at 400°C and 0.1 GPa: A Structural Mechanism for the Assembly of Cs<Subscript>2</Subscript>ZrSi<Subscript>6</Subscript>O<Subscript>15</Subscript> (C2/m) and Cs<Subscript>2</Subscript>TiSi<Subscript>6</Subscript>O<Subscript>15</Subscript> (C2/c) from Subpolyhedral Clusters, Zh. Neorg. Khim., 2003, vol. 48, no. 12, pp. 1955–1958.
Vukalovich, M.P., Teplofizicheskie svoistva vody i vodyanogo para (Thermophysical Properties of Water and Water Vapor), Moscow: Mashinostroenie, 1967.
Hazen, R.M. and Finger, L.W., Crystal Structure and Compressibility of Zircon at High Pressure, Am. Mineral., 1979, vol. 64, pp. 196–201.
Kohler, H., Schulz, H., and Mel'nikov, O.K., Structural Investigations of Nasicon (Na<Subscript>1</Subscript> + <Subscript>x</Subscript>Zr<Subscript>2</Subscript>Si<Subscript>x</Subscript>P<Subscript>3</Subscript>-xO<Subscript>12</Subscript>; x = 3) with X-ray Diffraction at 298 K and 403 K, Mater. Res. Bull., 1983, vol. 18, pp. 589–592.
Voronkov, A.A. and Pyatenko, Yu.A., Crystal Structure of Vlasovite, Kristallografiya, 1961, vol. 6, no. 6, pp. 937–943.
Voronkov, A.A., Shumyatskaya, N.G., and Pyatenko, Yu.A., On the Crystal Structure of a New Natural Na<Subscript>2</Subsrcipt>ZrSi<Subscript>2</Subscript>O<Subscript>7</Subscript> Polymorph, Zh. Strukt. Khim., 1970, vol. 11, no. 5, pp. 932–933.
Cannillo, E., Rossi, G., and Ungaretti, L., The Crystal Structure of Elpidite, Am. Mineral., 1973, vol. 58, pp. 106–109.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ilyushin, G.D. Hydrothermal Crystallization in the Na2CO3–ZrO2–SiO2–H2O System at 500°C and 0.1 GPa: Na2ZrSi4O11, Na2ZrSi2O7, and Na4Zr2Si3O12 Phase Relations. Inorganic Materials 40, 860–866 (2004). https://doi.org/10.1023/B:INMA.0000037934.80683.8f
Issue Date:
DOI: https://doi.org/10.1023/B:INMA.0000037934.80683.8f