Abstract
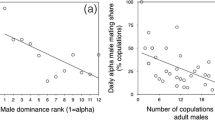
We investigated the costs of mating with multiple males in terms of feeding time, traveling distances, sexual proceptivity, and male aggression, for wild female (Macaca fuscata yakui) on Yakushima Island, Japan. We analyzed all-day focal sampling data from 7 females during the mating season (Sept.-Nov. 1996). On days when estrous females copulated with multiple males, they decreased their feeding time to half that of anestrous days, traveled longer distances, showed more proceptive sexual behaviors and received more aggression from subordinate males than on days when they copulated with only the 1st-ranking male. On days when females copulated with only the 1st-ranking male, they showed no difference in feeding time with that of anestrous days, and expended less effort than the above mating pattern because of short traveling distances, diminished sexual proceptivity and a lower frequency of aggression received. The results suggest that the costs of estrous vary according to female sexual proceptivity and the number and social status of mating partners. Female Japanese macaques exhibit a mixed mating strategy over prolonged estrous periods, which may provide females with opportunities to maximize the benefits of copulating with multiple males and to minimize the costs of estrus by mating with only the 1st-ranking male. During an estrous cycle, females may be adjusting efforts for reproduction and survival; i.e., mating vs. feeding.
Similar content being viewed by others
REFERENCES
Albert, S. C., Altmann, J., and Wilson, M. L. (1996). Mate guarding constrains foraging activity of male baboons. Anim. Behav. 51: 1269-1277.
Altmann, J. (1974). Observational study of behaviour: Sampling methods. Behaviour 49: 227-265.
Arnqvist, G. (1989). Multiple mating in a water strider: Mutual benefits or intersexual conflict? Anim. Behav. 38: 749-756.
Aujard, F., Heistermann, M., Thierry, B., and Hodges, J. K. (1998). Functional significance of behavioral morphological and endocrine correlates across the ovarian cycle in semifree ranging female Tonkean macaques. Am. J. Primatol. 46: 285-309.
Beach, F. A. (1976). Sexual attractivity proceptivity and receptivity in female mammals. Horm. and Behav. 7: 105-138.
Bercovitch, F. B. (1983). Time budgets and consortships in olive baboons (Papio anubis).Folia Primatol. 41: 180-190.
Bercovitch, F. B. (1987). Female weight and reproductive condition in a population of olive baboons (Papio anubis).Am. J. Primatol. 12: 189-195.
Bercovitch, F. B., Lebron, M. R., Martinez, H. S., and Kessler, M. J. (1998). Primigravidity body weight and costs of rearing first offspring in rhesus macaques. Am. J. Primatol. 46: 135-144.
Bielert, C., and Busse, C. (1983). Influences of ovarian hormones on the food intake and feeding of captive and wild female chacma baboons (Papio ursinus).Physiol. Behav. 30: 103-111.
Brereton, A. R. (1992). Alternative reproductive tactics in stumptail macaques (Macaca arctoides).Folia Primatol. 59: 209-212.
Brereton, A. R. (1994). Return-benefit spite hypothesis: An explanation for sexual interference in stumptail macaques (Macaca arctoides).Primates 35: 123-136.
Bulger, J. B. (1993). Dominance rank and access to estrous females in male savanna baboons. Behaviour 127: 67-103.
Chapman, T., Liddle, L. F., Kalb, J. M., Wolfner, M. F., and Partridge, L. (1995). Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373: 241-244.
Czaja, J. A., and Goy, R. W. (1975). Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm. Behav. 6: 329-349.
Daly, M. (1978). The cost of mating. Am. Nat. 112: 771-774.
Dixson, A. F. (1997). Evolutionary perspectives on primate mating systems and behavior. Ann. N. Y. Acad. Sci. 807: 42-61.
Dixson, A. F. (1998). Primate Sexuality, Oxford University Press, New York.
Dwass, M. (1960). Some k-sample rank-order tests. In Olkin, I. (ed.), Contributions to Probability and Statistics Stanford University Press, California, pp. 198-202.
Enomoto, T. (1974). The sexual behavior of Japanese monkeys. J. Hum. Evol. 3: 351-372.
Enomoto, T. (1979). On the correlation between sexual behavior and ovarian hormone level during the menstrual cycle in captive Japanese monkeys. Primates 20: 563-570.
Fedigan, L. M. (1983). Dominance and reproductive success in primates. Yrbk Phys. Anthropol. 26: 91-129.
Furuichi, T. (1985). Inter-male associations in a wild Japanese macaque troop on Yakushima Island Japan. Primates 26: 219-237.
Furuichi, T. (1987). Sexual swelling receptivity and grouping of wild pygmy chimpanzee females at Wamba Zaire. Primates 28: 309-318.
Hasegawa, T. (1990). Sex differences in ranging patterns. In Nishida, T (ed.), The Chimpanzees of the Mahale Mountains, University of Tokyo Press, Tokyo, pp. 99-114.
Hausfater, G. (1975). Dominance and Reproduction in Baboons (Papio cynocephalus): A Quantitative Analysis, S. Karger, Basel, Switzerland.
Hogg, J. T. (1984). Mating in bighorn sheep: Multiple creative male strategies. Science 225: 526-528.
Hrdy, S. B. (1977). Infanticide as a primate reproductive strategy. Am. Sci. 65: 40-49.
Huffman, M. A. (1987). Consort intrusion and female male choice in Japanese macaques (Macaca fuscata).Ethology 75: 221-234.
Huffman, M. A. (1991). Mate selection and partner preference in female Japanese macaques. In Fedigan, L. M., and Asquith, P. (eds.), The Monkeys of Arashiyama. Thirty-Five Years of Study in the East and West, State University of New York Press, Albany, pp. 101-122.
Huffman, M. A. (1992). Influences of female partner preference on potential reproductive outcome in Japanese macaques. Folia Primatol. 59: 77-88.
Kurland, J. A. (1977). Kin selection in the Japanese monkey. In Szalay, E. S. (ed.), Contributions to Primatology, Vol. 12, S. Karger, New York, pp. 1-145.
Manson, J. H. (1997). Primate consortships. Curr. Anthropol. 38: 353-374.
Maruhashi, T. (1980). Feeding behavior and diet of the Japanese monkeys (Macaca fuscata yakui) on Yakushima Island Japan. Primates 21: 141-160.
Maruhashi, T. (1982). An ecological study of troop fissions of Japanese monkeys (Macaca fuscata yakui) on Yakushima Island Japan. Primates 23: 317-337.
Matsubara, M. (2003). Costs of mate guarding and opportunistic mating among wild male Japanese macaques. Int. J. Primatol. 24: 1057-1075.
Matsumoto-Oda, A., and Oda, R. (1998). Changes in the activity budget of cycling female chimpanzees. Am. J. Primatol. 46: 157-166.
Milton, K. (1985). Mating patterns of wooly spider monkeys Brachyteles arachnoides: Implications for female choice. Behav. Ecol. Sociobiol. 17: 53-59.
Mitsunaga, F., Nozaki, M., Inoue, M., Takenaka, A., Takenaka, O., Sakura, O., Sugiyama, Y., and Ohsawa, H. (1992). Steroid hormones and sexual behavior of female Japanese monkeys in an enclosed group. In Itoigawa, N., Sugiyama, Y., Sackett, G. P., and Thompson, R. K. R. (eds.), Topics in Primatology 2, University of Tokyo Press, Tokyo, pp. 23-34.
Mori, A., Yamaguchi, N., Watanabe, K., Shimizu, K. (1997). Japanese macaques under poor nutritional conditions and food-enhanced perineal swelling in the Koshima troop. Int. J. Primatol. 18: 553-579.
Nunn, C. L. (1999). The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim. Behav. 58: 229-246.
Okayasu, N. (1992). Prolonged estrus in female Japanese macaques (Macaca fuscata yakui) and the social influence on estrus: With special reference to male intertroop movement. In Itoigawa, N., Sugiyama, Y., Sackett, G. P., and Thompson, R. K. R. (eds.), Topics in Primatology 2, University of Tokyo Press, Tokyo, pp. 163-178.
Parker, G. A. (1984). Human sperm competition. In Smith, R. L. (ed.), Sperm Competition and the Evolution of Animal Mating Systems, Academic Press, Orlando, Tokyo, pp. 601-659.
Paul, A. (2002). Sexual selection and mate choice. Int. J. Primatol. 23: 877-904.
Reynolds, J. D., and Gross, M. R. (1990). Costs and benefits of female mate choice: Is there a lek paradox? Am. Nat. 136: 230-243.
Saayman, G. S. (1970). The menstrual cycle and sexual behavior in a troop of free ranging Chacma baboons (Papio ursinus).Folia. Primatol. 12: 81-110.
Small, M. F. (1988). Female primate sexual behavior and conception: Are there really sperm to spare? Curr. Anthropol. 29: 81-100.
Smuts, B. B. (1987). Sexual competition and mate choice. In Smuts, B. B., Cheney, D. L., Seyfarth, R. M., Wrangham, R. W., and Struhsaker, T. T. (eds.), Primate Societies, University of Chicago Press, Chicago, pp. 385-399.
Smuts, B. B., and Smuts, R. W. (1993). Male aggression and sexual coercion of females in nonhuman primates and other mammals: Evidence and theoretical implications. Adv. Stud. Behav. 22: 1-63.
Soltis, J. (1999). Measuring male–female relationships during the mating season in wild Japanese macaques (Macaca fuscata yakui).Primates 40: 453-467.
Soltis, J. (2002). Do primate female gain nonprocreative benefits by mating with multiple males? Theoretical and empirical considerations. Evol. Anthropol. 11: 187-197.
Soltis, J., and McElreath, R. (2001). Can females gain extra paternal investment by mating with multiple males? A game theoretic approach. Am. Nat. 158: 519-529.
Soltis, J., Mitsunaga, F., Shimizu, K., Nozaki, M., Yanagihara, Y., Domingo-Roura, X., and Takenaka, O. (1997a). Sexual selection in Japanese macaques I: Female mate choice and male–male competition. Anim. Behav. 54: 725-736.
Soltis, J., Mitsunaga, F., Shimizu, K., Nozaki, M., Yanagihara, Y., Domingo-Roura, X., and Takenaka, O. (1997b). Sexual selection in Japanese macaques II: Female mate choice and male–male competition. Anim. Behav. 54: 737-746.
Sprague, D. S. (1998). Topographic effects on measures of monkey habitat-use in a mountainous study site in Japan. Am. J. Phys. Anthropol. 26: 207.
Sprague, D. S., Suzuki, S., Takahashi, H., and Sato, S. (1998). Male life history in natural populations of Japanese macaques: Migration, dominance rank, and troop participation of males in two habitats. Primates 39: 351-363.
Steel, R. D. G. (1960). A rank sum test for comparing all pairs of treatments. Technometrics 2: 197-207.
Sugiyama, Y. (1965). On the social charge of hanuman langurs (Presbytis entellus) in their natural condition. Primates 6: 213-247.
Takahata, Y. (1980). The reproductive biology of a free-ranging troop of Japanese monkeys. Primates 21: 303-329.
Takahata, Y., Huffman, M. A., Suzuki, S., Koyama, N., and Yamagiwa, J. (1999). Why dominants do not consistently attain high mating and reproductive success: A review of longitudinal Japanese macaque studies. Primates 40: 143-158.
Tardif, S. D., and Jaquish, C. E. (1994). The common marmoset as a model for nutritional impacts upon reproduction. Ann. N. Y. Acad. Sci. 709: 214-215.
Taub, D. M. (1980). Female choice and mating strategies among wild Barbary macaques (Macaca sylvanus).In Lindburg, D. G. (ed.), The Macaques: Studies in Ecology, Behavior and Evolution, Van Nostrand Reinhold, New York, pp. 287-344.
Taylor, C. R., Schmidt-Nielsen, K., and Raab, J. L. (1970). Scaling of energetic cost of running to body size in mammals. Am. J. Physiol. 219: 1104-1107.
Tutin, C. E. G. (1979). Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii).Behav. Ecol. Sociobiol. 6: 29-38.
van Noordwijk, M. A., and van Schaik, C. P. (2000). Reproductive patterns in eutherian mammals: Adaptations against infanticide? In Schaik, C. P., and Janson, C. H. (eds.), Infanticide by Males and its Consequences, Cambridge University Press, Cambridge, pp. 322-360.
Wada, K., Moriya, K., Hara, K., and Ohsawa, W. (1975). On the body fat of Japanese monkeys inhabiting the Shiga Heights (in Japanese with English summary). Physiol. Ecol. Jpn. 16: 104-107.
Walker, M. L., Gordon, T. P., and Wilson, M. E. (1983). Menstrual cycle characteristics of seasonally breeding rhesus monkeys. Biol. Reprod. 29: 841-848.
Wrangham, R. W. (1979). Sex differences in chimpanzee dispersion. In Hamburg, D. A., and McCown, E. R. (eds.), The Great Apes, Benjamin/Cummings Publication, Menlo Park, CA, pp. 481-489.
Yamagiwa, J. (1985). Socio-sexual factors of troop fission in wild Japanese monkeys (Macaca fuscata yakui) Yakushima Island Japan. Primates 26: 105-120.
Yamagiwa, J., and Hill, D. A. (1998). Intraspecific variation in the social organization of Japanese macaques: Past and present scope of field studies in natural habitats. Primates 39: 257-273.
Yasui, Y. (1998). The ‘genetic benefits’ of female multiple mating reconsidered. Trends Ecol. Evol. 13: 246-250.
Yoshihiro, S., Ohtake, M., Matsubara, H., Zamma, K., Hanya, G., Tanimura, Y., Kubota, H., Kubo, R., Arakane, T., Hirata, T., Furukawa, M., Sato, A., and Takahata, Y. (1999). Vertical distribution of wild Yakushima macaques (Macaca fuscata yakui) in the western area of Yakushima Island Japan: Preliminary report. Primates 40: 409-415.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsubara, M., Sprague, D.S. Mating Tactics in Response to Costs Incurred by Mating with Multiple Males in Wild Female Japanese Macaques. International Journal of Primatology 25, 901–917 (2004). https://doi.org/10.1023/B:IJOP.0000029128.00559.91
Issue Date:
DOI: https://doi.org/10.1023/B:IJOP.0000029128.00559.91