Abstract
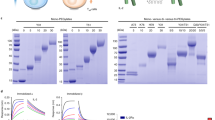
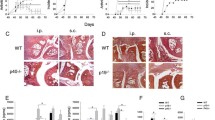
Experiments with immunostimulatory unmethylated CpG-containing DNA are usually conducted with nuclease-protected phosphorothioate oligodeoxynucleotides (S-ODNs), rather than phosphodiester oligodeoxynucleotides (O-ODNs). We compared the murine immune responses to S-ODNs and O-ODNs that either contained or lacked CpG motifs. Both CpG and non-CpG S-ODNs induced synovitis, as did sequence-matched CpG O-ODN, but not GpC O-ODN. There was a minimum length requirement for arthritogenic S-ODNs since a CpC dinucleotide S-ODN did not induce arthritis. There were both sequence-(CpG > non-CpG) and backbone-dependent (S-ODN > O-ODN) differences in the levels of DNA-induced arthritis upon intra-articular injection with the ODNs. However, CpG O-ODN being an exception, induced more severe arthritis than the GpC S-ODN. The levels of in vitro proliferation and production of IL-6, TNF-α, IL-12, and RANTES by splenocytes following exposure to CpG S-ODN were significantly higher than those induced by CpG O-ODN. In addition, both proliferative responses and cytokine production induced by S-ODN-stimulated splenocytes increased significantly when the S-ODN contained a CpG motif. Transcription factor NFκB was activated by both CpG S-ODN and CpG O-ODN but interestingly not by GpC S-ODN. This indicates that the NFκB signal pathway modulates CpG-mediated immunostimulation, while sequence-independent immune activation by the phosphorothioate backbone is probably signalled via a different pathway.
Similar content being viewed by others
REFERENCES
Hemmi, H, O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshing, H. Wagner, K. Takeda, and S. Akira. 2000. A toll-like receptor recognizes bacterial DNA. Nature 408: 740-745.
Krieg, A. M. 2000. The role of CpG motifs in innate immunity. Curr. Opin. Immunol. 12: 35-43.
Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374: 546-549.
Moldoveanu, Z., L. Love-Homan, W. Q. Huang, and A. M. Krieg. 1998. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine 16: 1216-1224.
Han, S. S., S. T. Chung, D. A. Robertson, R. L. Chelvarajan, and S. Bondada. 1999. CpG oligodeoxynucleotides rescue BKS-2 immature B cell lymphoma from anti-IgM-mediated growth inhibition by up-regulation of egr-1. Int. Immunol. 11: 871-879.
Krieg, A. M., T. Wu, R. Weeratna, S. M. Efler, L. Love-Homan, L. Yang, A. K. Yi, D. Short, H. L. Davis. 1998. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc. Natl. Acad. Sci. U.S.A. 95: 12631-12636.
Yi, A. K. and A. M. Krieg. 1998. CpG DNA rescue from anti-IgM-induced WEHI-231 B lymphoma apoptosis via modulation of I kappa B alpha and I kappa B beta and sustained activation of nuclear factor-kappa B/c-Rel. J. Immunol. 160: 1240-1245.
Yi, A. K., D. W. Peckham, R. F. Ashman, and A. M. Krieg. 1999. CpG DNA rescues B cells from apoptosis by activating NFkappaB and preventing mitochondrial membrane potential disruption via a chloroquine-sensitive pathway. Int. Immunol. 11: 2015-2024.
Qi, L. and K. H. Sit. 2000. CpG-specific common commitment in caspase-dependent and-independent cell deaths. Mol. Cell. Biol. Res. Commun. 3: 33-41.
Sparwasser, T., T. Miethke, G. Lipford, A. Erdmann, H. Hacker, K. Heeg, and H. Wagner. 1997. Macrophages sense pathogens via DNA motifs: Induction of tumor necrosis factor-alpha-mediated shock. Eur. J. Immunol. 27: 1671-1679.
Sester, D. P., S. Naik, S. J. Beasley, D. A. Hume, and K. J. Stacey. 2000. Phosphorothioate backbone modification modulates macrophage activation by CpG DNA. J. Immunol. 165: 4165-4173.
Halpern, M. D. and D. S. Pisetsky. 1995. In vitro inhibition of murine IFN gamma production by phosphorothioate deoxyguanosine oligomers. Immunopharmacology 29: 47-52.
Bauer, M., K. Heeg, H. Wagner, and G. B. Lipford. 1999. DNA activates human immune cells through a CpG sequence-dependent manner. Immunology 97: 699-705.
Liang. H., Y. Nishioka, C. F. Reich, D. S. Pisetsky, and P. E. Lipsky. 1996. Activation of human B cells by phosphorothioate oligodeoxynucleotides. J. Clin. Invest. 98: 1119-1129.
Parronchi, P., F. Brugnolo, F. Annunziato, C. Manuelli, S. Sampognaro, C. Mavilia, S. Romagnani, and E. Maggi. 1999. Phosphorothioate oligodeoxynucleotides promote the in vitro development of human allergen-specific CD4+ T cells into Th1 effectors. J. Immunol. 163: 5946-5953.
Yew, N. S., K. X. Wang, M. Przybylska, R. G. Bagley, M. Stedman, J. Marshall, R. K. Scheule, and S. H. Cheng. 1999. Contribution of plasmid DNA to inflammation in the lung after administration of cationic lipid: pDNA complexes. Hum. Gene. Ther. 10: 223-234.
Yew, N. S., H. Zhao, I. H. Wu, A. Song, J. D. Tousignant, M. Przybylska, S. H. Cheng. 2000. Reduced inflammatory response to plasmid DNA vectors by elimination and inhibition of immunostimulatory CpG motifs. Mol. Ther. 1: 255-262.
Li, S., S. P. Wu, M. Whitmore, E. J. Loeffert, L. Wang, S. C. Watkins, B. R. Pitt, L. Huang. 1999. Effect of immune response on gene transfer to the lung via systemic administration of cationic lipidic vectors. Am. J. Physiol. 276: L796-L804.
Schwartz, D. A., T. J. Quinn, P. S. Thorne, S. Sayeed, A. K. Yi, and A. M. Krieg. 1997. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J. Clin. Invest. 100: 68-73.
Takeshita, S., F. Takeshita, D. E. Haddad, K. J. Ishii, and D. M. Klinman. 2000. CpG oligodeoxynucleotides induce murine macrophages to up-regulate chemokine mRNA expression. Cell Immunol. 206: 101-106.
Deng, G. M., I. M. Nilsson, M. Verdrengh, L. V. Collins, and A. Tarkowski. 1999. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat. Med. 5: 702-705.
Miyata, M., H. Kobayashi, T. Sasajima, Y. Sato, and R. Kasukawa. 2000. Unmethylated oligo-DNA containing CpG motifs aggravates collagen-induced arthritis in mice. Arthritis Rheum. 43: 2578-2582.
Lee, S. W., M. K. Song, K. H. Baek, Y. Park, J. K. Kim, C. H. Lee, H. K. Cheong, C. Cheong, and Y. C. Sung. 2000. Effects of a hexameric deoxyriboguanosine run conjugation into CpG oligodeoxynucleotides on their immunostimulatory potentials. J. Immunol. 165: 3631-3639.
Dalpke, A. H., S. Zimmermann, I. Albrecht, and K. Heeg. 2002. Phosphodiester CpG oligonucleotides as adjuvants: Polyguanosine runs enhance cellular uptake and improve immunostimulative activity of phosphodiester CpG oligonucleotides in vitro and in vivo. Immunology 106: 102-112.
Kline, J. N., T. J. Waldschmidt, T. R. Businga, J. E. Lemish, J. V. Weinstock, P. S. Thorne, and A. M. Krieg. 1998. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J. Immunol. 160: 2555-2559.
Broide, D., J. Schwarze, H. Tighe, T. Gifford, M. D. Nguyen, S. Malek, J. Van Uden, E. Martin-Orozco, E. W. Gelfand, and E. Raz. 1998. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J. Immunol. 161: 7054-7062.
Sur, S., J. S. Wild, B. K. Choudhury, N. Sur, R. Alam, and D. M. Klinman. 1999. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J. Immunol. 162: 6284-6293.
Bremell, T., A. Abdelnour, and A. Tarkowski. 1992. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect. Immun. 60: 2976-2985.
Bergquist, J., B. Ohlsson, and A. Tarkowski. 2000. Nuclear factor-kappa B is involved in the catecholaminergic suppression of immunocompetent cells. Ann. N. Y. Acad. Sci. 917: 281-289.
Krieg, A. M. 2001. Now I know my CpGs. Trends Microbiol. 9: 249-252.
Sweet, M. J., C. C. Campbell, D. P. Sester, D. Xu, R. C. McDonald, K. J. Stacey, D. A. Hume, and F. Y. Liew. 2002. Colony-stimulating factor-1 suppresses responses to CpG DNA and expression of toll-like receptor 9 but enhances responses to lipopolysaccharide in murine macrophages. J. Immunol. 168: 392-399.
Jakob, T., P. S. Walker, A. M. Krieg, M. C. Udey, and J. C. Vogel. 1998. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: A role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J. Immunol. 161: 3042-3049.
Podlaski, F. J., V. B. Nanduri, J. D. Hulmes, Y. C. Pan, W. Levin, W. Danho, R. Chizzonite, M. K. Gately, and A. S. Stern. 1992. Molecular characterization of interleukin 12. Arch. Biochem. Biophys. 294: 230-237.
Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16: 225-260.
Katrib, A., H. P. McNeil, and P. P. Youssef. 2002. What can we learn from the synovium in early rheumatoid arthritis? Inflamm. Res. 51: 170-175.
Trinchieri, G. 1998. Proinflammatory and immunoregulatory functions of interleukin-12. Int. Rev. Immunol. 16: 365-396.
Deng, G. M. and A. Tarkowski. 2001. Synovial cytokine mRNA expression during arthritis triggered by CpG motifs of bacterial DNA. Arthritis. Res. 3: 48-53.
Van Snick, J. 1990. Interleukin-6: An overview. Annu. Rev. Immunol. 8: 253-278.
Ohshima, S., Y. Saeki, T. Mima, M. Sasai, K. Nishioka, S. Nomura, M. Kopf, Y. Katada, T. Tanaka, M. Suemura, and T. Kishimoto. 1998. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc. Natl. Acad. Sci. U.S.A. 95: 8222-8226.
Igaz, P., A. Horvath, B. Horvath, C. Szalai, E. Pallinger, E. Rajnavolgyi, S. Toth, S. Rose-John, and A. Falus. 2000. Soluble interleukin-6 receptor (sIL-6R) makes IL-6R negative T cell line respond to IL-6; it inhibits TNF production. Immunol. Lett. 71: 143-148.
Aderka, D., J. M. Le, and J. Vilcek. 1989. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J. Immunol. 143: 3517-3523.
Volin, M. V., M. R. Shah, M. Tokuhira, G. K. Haines, J. M. Woods, and A. E. Koch. 1998. RANTES expression and contribution to monocyte chemotaxis in arthritis. Clin. Immunol. Immunopathol. 89: 44-53.
Deng, G. M. and A. Tarkowski. 2000. The features of arthritis induced by CpG motifs in bacterial DNA. Arthritis. Rheum. 43: 356-364.
Zhao, Q., J. Temsamani, P. L. Iadarola, Z. Jiang, and S. Agrawal. 1996. Effect of different chemically modified oligodeoxynucleotides on immune stimulation. Biochem. Pharmacol. 51: 173-182.
Matson, S. and A. M. Krieg. 1992. Nonspecific suppression of '3H'thymidine incorporation by “control” oligonucleotides. Antisense Res. Dev. 2: 325-330.
Zhao, Y. X., A. Abdelnour, R. Holmdahl, and A. Tarkowski. 1995. Mice with the xid B cell defect are less susceptible to developing Staphylococcus aureus-induced arthritis. J. Immunol. 155: 2067-2076.
Deng, G. M., M. Verdrengh, and Z. Q. Liu, A. Tarkowski. 2000. The major role of macrophages and their product tumor necrosis factor alpha in the induction of arthritis triggered by bacterial DNA containing CpG motifs. Arthritis. Rheum. 43: 2283-2289.
Molne, L., L. V. Collins, and A. Tarkowski. Inflammatogenic properties of bacterial DNA following cutaneous exposure. J. Invest. Dermatol. 121: 294-299.
Takeshita, F. and D. M. Klinman. 2000. CpG ODN-mediated regulation of IL-12 p40 transcription. Eur. J. Immunol. 30: 1967-1976.
Takeshita, F., K. J. Ishii, A. Ueda, Y. Ishigatsubo, and D. M. Klinman. 2000. Positive and negative regulatory elements contribute to CpG oligonucleotide-mediated regulation of human IL-6 gene expression. Eur. J. Immunol. 30: 108-116.
Mason, N., J. Aliberti, J. C. Caamano, H. C. Liou, and C. A. Hunter. 2002. Cutting edge: Identification of c-Rel-dependent and-independent pathways of IL-12 production during infectious and inflammatory stimuli. J. Immunol. 168: 2590-2594.
Hartmann, G. and A. M. Krieg. 2000. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol. 164: 944-953.
Yi, A. K. and A. M. Krieg. 1998. Rapid induction of mitogen-activated protein kinases by immune stimulatory CpG DNA. J. Immunol. 161: 4493-4497.
Hacker, H., H. Mischak, T. Miethke, S. Liptay, R. Schmid, T. Sparwasser, K. Heeg, G. B. Lipford, and H. Wagner. 1998. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. Embo. J. 17: 6230-6240.
Zhao, Q., S. Matson, C. J. Herrera, E. Fisher, H. Yu, and A. M. Krieg. 1993. Comparison of cellular binding and uptake of antisense phosphodiester, phosphorothioate, and mixed phosphorothioate and methylphosphonate oligonucleotides. Antisense Res. Dev. 3: 53-66.
Chuang, T. H., J. Lee, L. Kline, J. C. Mathison, and R. J. Ulevitch. 2002. Toll-like receptor 9 mediates CpG-DNA signaling. J. Leukoc. Biol. 71: 538-544.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bjersing, J.L., Eriksson, K., Tarkowski, A. et al. The Arthritogenic and Immunostimulatory Properties of Phosphorothioate Oligodeoxynucleotides Rely on Synergy Between the Activities of the Nuclease-Resistant Backbone and CpG Motifs. Inflammation 28, 39–51 (2004). https://doi.org/10.1023/B:IFLA.0000014710.44475.94
Issue Date:
DOI: https://doi.org/10.1023/B:IFLA.0000014710.44475.94