Abstract
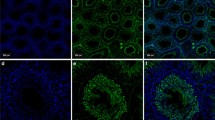
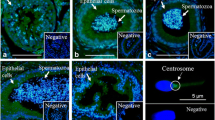
Spermatozoa are subjected to major changes as they pass through the epididymal duct. The aim of the present study was to describe the distribution of carbonic anhydrase (CA) in the mouse testis and epididymis using a histochemical technique showing total catalytic activity, in combination with immunohistochemistry for the two important isoforms CAs II and IV. By comparing normal mice with CA II-deficient mice, we were able to study membrane-bound CA without influence from the ubiquitous cytoplasmic CA II. Spermatozoa, when studied in both the scanning electron and light microscope, were found to pickup membrane-bound CA IV during their passage through the epididymal duct. The transfer appeared to take place in the proximal part of the corpus, where the apical membrane and vesicles of principal cells were richly supplied with CA IV. In addition to CA IV, another membrane-bound isozyme was located in basolateral membranes of principal cells. Cytoplasmic CA II was found in varying amounts in apical/narrow cells and principal cells of the corpus in control animals. The significance of CA for pH-regulating processes vital for sperm storage and motility is discussed. A function in HCO3 − transport during sperm capacitation at fertilization is suggested for the CA IV found in spermatozoa.
Similar content being viewed by others
References
Aberdam E, Romero C, Ortonne JP (1993) Repeated UVBirradiations do not have the same potential to promote stimulation of melanogenesis in cultured normal melanocytes. J Cell Sci 106: 1015–1022.
Bors W, Heller W, Michel C, Saran M(1990) Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Meth Enzymol 186: 343–355.
Busam KJ, Iversen K, Coplan KA, Old LJ, Stockert E, Chen YT, McGergor D, Jungbluth A (1998) Immunoreactivity for A103, an antibody to Melan-A (MART-1), in adrenocortical and other steroid tumors. Am J Surg Path 22: 57–63.
Chen QX, Kubo I (2002) Kinetics of mushroom tyrosinase inhibition by quercetin. J Agric Food Chem 50: 4108–4012.
Chen YT, Stockert E, Jungbluth A, Tsang S, Coplan KA, Scanlan MJ, Old LJ (1996) Serological analysis of Melan-A (MART-1), a melanocyte-specific protein homogeneously expressed in human melanomas. Proc Natl Acad Sci USA 93: 5915–5919.
Erden IM, Kahraman A, Koken T (2001) Beneficial effect of quercetin on oxidative stress induced by ultraviolet A. Clin Exp Dermatol 26:536–539.
Friedmann PS, Gilchrest BA(1987) Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol 133: 88–94.
Gordon PR, Mansur CP, Gilchrest BA (1989) Regulation of human melanocyte growth, dendricity and melanization by keratinocyte derived factors. J Invest Dermatol 92: 565–572.
Hearing VJ, Korner AM, Pawelek JM (1982) New regulators of melano-genesis are associated with purified tyrosinase isozymes. J Invest Dermatol 79: 16–18.
Imokawa G, Miyagishi M, Yada Y (1995) Endothelin-1 as a new melanogen: Coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol 105: 32–37.
Katsarou A, Davoy E, Xenos K, Armenaka M, Theoharides TC (2000) Effect of an antioxidant (quercetin) on sodium-lauryl-sulfate-induced skin irritation. Contact Dermatitis 42: 85–89.
Kindblom LG, Lodding P, Rosengren L, Baudier J, Haglid K (1984) S-100 protein in melanocytic tumors. An immunohistochemical investigation of benign and malignant melanocytic tumors and metastases of malignant melanoma and a characterization of the anti-gen in comparison to human brain. Acta Pathol Microbiol Immunol Scand Sect A 92: 219–230.
Lacour JP, Gordon PR, Eller M, Bhawan J, Gilchrest BA (1992) Cytoskeleton events underlying dendrite formation by cultured pigment cells. J Cell Physiol 151: 287–299.
Laemimli UK(1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Laskin JD, Piccinini LA (1986) Tyrosinase isozyme heterogeneity in differentiating B16/C3 melanoma. J Biol Chem 261: 16226–16235.
Monteiro-Riviere N, Inman AO, Snider TH, Blank J, Hobson DW(1997) Comparison of an in vitro skin model to normal human skin for dermatological research. Microsc Res.Tech 37: 172–179.
Nagata H, Takekoshi S, Takagi T, Honma T, Watanabe K (1999) Antioxidative action of flavonoids, quercetin and catechin, mediated by the activation of glutathione peroxidase. Tokai J Exp Clin Med 24: 1–11.
Naeyaert JM, Eller M, Gordon PR, Park HY, Gilchrest BA (1991) Pigment content of cultured human melanocytes does not correlate with tyrosinase message level. Br J Dermatol 125: 297–303.
Nguyen TD, Canada AT, Heintz GG, Gettys TW, Cohn JA (1991) Stimulation of secretion by the T84 colonic epithelial cell line with dietary flavonols. Biochem Pharmacol 41: 1879–1886.
Ree K (1983) Increased size of stages II and III melanosomes during PUVA therapy. Acta Derm Venereol 63: 15–20.
Rosenthal MH, Kreider JW Shiman R (1973) Quantitative assay of melanin in melanoma cell in culture and in tumor. Anal Biochem 56: 91–99.
Sato N, Suzuki S, Takimoto H, Masui S, Shibata K, Nakano H, Tomita Y (1996) Monoclonal antibody MAT-1 against human tyrosinase can detect melanogenic cells on formalin-fixed paraffin-embedded section. Pigment Cell Res 9: 72–76.
Scott G (2002) Rac and Rho: The story behind melanocyte dendrite formation. Pigment Cell Res 15: 322–330.
Staricco RJ, Pinkus H (1957) Quantitative and qualitative data on the pigment cells of adult human epidermis. J Invest Dermatol 28: 33–45.
Takagi T, Takekoshi S, Okabe T, Nagata H, Honma T, Watanabe K (1998) Quercetin, a flavonol, promotes disassembly of microtubules in prostate cancer cells: Possible mechanism of its antitumor activity. Acta Histochem Cytochem 31: 435–445.
Takimoto H, Suzuki S, Masui S, Shibata K, Tomita Y, Shibahara S, Nakano H (1995) MAT-1, a monoclonal antibody that specifically recognized human tyrosinase. J Invest Dermatol 105: 764–768.
Towbin H, Staehein T, Gordon J (1989) Electrophoretic transfer of proteins from polyacrylamide gels to nitro-cellulose sheets: Procedure and soma applications. Proc Natl Acad Sci USA 76: 4350–4354.
Zhao JF, Zhang YJ, Kubilus J, Jin XH, Santella RM, Athar M, Wang ZY, Bickers DR(1999) Reconstituted 3-dimensional human skin as a novel in vitro model for studies of carcinogenesis. Biochem Biophys Res Commun 254: 49–53.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ekstedt, E., Holm, L. & Ridderstråle, Y. Carbonic Anhydrase in Mouse Testis and Epididymis; Transfer of Isozyme IV to Spermatozoa During Passage. Histochem J 35, 167–173 (2004). https://doi.org/10.1023/B:HIJO.0000023387.02793.af
Issue Date:
DOI: https://doi.org/10.1023/B:HIJO.0000023387.02793.af