Abstract
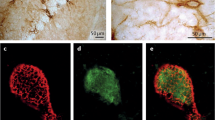
Perineuronal nets (PNs) of the extracellular matrix have been shown to develop in organotypic slice cultures largely corresponding with regional patterns known from in vivo experiments. In the present study, we use vital labelling to investigate aspects of the cell type-dependent development of PNs associated with nonpyramidal neurons and pyramidal cells in the parietal cortex and hippocampus. Frontal sections were cut from brains of 3–5-day-old rats and were cultured for 3–5 weeks. PNs were sequentially labelled using biotinylated Wisteria floribunda agglutinin and chromogen-tagged streptavidin either in living slice cultures, examined by confocal microscopy in vitro, or in cultures examined by confocal and electron microscopy after fixation. Nonpyramidal and pyramidal cells were characterized by immunoreaction for parvalbumin and the ionotropic glutamate receptor subunits 2/3. Vital labelling and examination of fixed slices correspondingly revealed that large numbers of PNs developed around cortical and hippocampal interneurons under depolarizing conditions induced by elevated external potassium concentration. After culture in standard medium, PNs were mainly found in association with subpopulations of pyramidal cells in the parietal cortex. PNs showed ultrastructural characteristics resembling those known from perfusion-fixed brain. A zone of labelled extracellular matrix aggregates was found in close proximity to the neuronal cell surface, surrounding presynaptic boutons and preterminal axons. The results show that characteristic features of PNs are retained after vital labelling in slice cultures. Moreover, our findings suggest that the cell type-specific development of PNs is regulated by patterns of intrinsic activity mediated by intra-cortical and -hippocampal synaptic contacts on potentially net-associated neurons.
Similar content being viewed by others
References
Adams I, Brauer K, Ar´elin C, H¨artig W, Fine A, M¨ader M, Arendt T, Br¨uckner G (2001) Perineuronal nets in the rhesus monkey and human basal forebrain including basal ganglia. Neuroscience 108: 285–298.
Annis CM, Robertson RT, O'Dowd DK (1993) Aspects of early post-natal development of cortical neurons that proceed independently of normally present extrinsic influences. J Neurobiol 24: 1460–1480.
Atoji Y, Hori Y, Sugimura M, Suzuki Y (1989) Extracellular matrix of the superior olivary nuclei in the dog. J Neurocytol 18: 599–610.
Baker RE, Bingmann D, Ruijter JM (1989) Electrophysiological properties of neurons in neonatal rat cortex slices grown in a serum-free medium. Neurosci Lett 97: 310–315.
Bertolotto A, Rocca G, Cavanese G, Migheli G, Schiffer D (1991) Chondroitin sulfate proteoglycan surrounds a subset of human and rat CNS neurons. J Neurosci Res 29: 225–234.
Bolz J, Novak N, G¨otz M, Bonhoeffer T (1990) Formation of target-specific projections in organotypic slice cultures from rat visual cortex. Nature 346: 359–362.
Bolz J, Novak N, Staiger V (1992) Formation of specific afferent connections in organotypic slice cultures from rat visual cortex cocultured with lateral geniculate nucleus. J Neurosci 12: 3054–3070.
Brauer K, Br¨uckner G, Leibnitz L, Werner L (1984) Structural and cytochemical features of perineuronal glial nets in the rat brain. Acta Histochem 74: 53–60.
Brauer K, H¨artig W, Bigl V, Br¨uckner G (1993) Distribution of parvalbumin-containing neurons and lectin-binding perineuronal nets in the rat basal forebrain. Brain Res 631: 167–170.
Br¨uckner G, Brauer K, H¨artig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A (1993) Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8: 183–200.
Br¨uckner G, Bringmann A, K¨oppe G, H¨artig W, Brauer K(1996a) In vivo and in vitro labelling of perineuronal nets in rat brain. Brain Res 720:84–92.
Br¨uckner G, Grosche J (2001) Perineuronal nets show intrinsic patterns of extracellular matrix differentiation in organotypic slice cultures. Exp Brain Res 137: 83–93.
Br¨uckner G, Grosche J, Hartlage-R¨ubsamen M, Schmidt S, Schachner M (2003) Region and lamina-specific distribution of extracellular matrix proteoglycans, hyaluronan and tenascin-R in the mouse hippocampal formation. J Chem Neuroanat 26: 37–50.
Br¨uckner G, Grosche J, Schmidt S, H¨artig W, Margolis RU, Delpech B, Seidenbecher CI, Czaniera R, Schachner M(2000) Postnatal develop-ment of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol 428: 616–629.
Br¨uckner G, Hausen D, H¨artig W, Drlicek M, Arendt T, Brauer K (1999) Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer's disease. Neuroscience 92: 791–805.
Br¨uckner G, H¨artig W, Kacza J, Seeger J, Welt K, Brauer K (1996b) Extracellular matrix organization in various regions of rat brain grey matter. J Neurocytol 25: 333–346.
Br¨uckner G, Seeger G, Brauer K, H¨artig W, Kacza J, Bigl V (1994) Cortical areas are revealed by distribution patterns of proteoglycan components and parvalbumin in the Mongolian gerbil and rat. Brain Res 658: 67–86.
Buchs P-A, Stoppini L, Muller D (1993) Structural modifications associated with synaptic development in area CA1 of rat hippocampal organotypic cultures. Dev Brain Res 71: 81–91.
Caeser M, Aertsen A (1991) Morphological organization of rat hippocampal slice cultures. J Comp Neurol 307: 87–106.
Caeser M, Bonhoeffer T, Bolz J (1989) Cellular organization and development of slice cultures from rat visual cortex. Exp Brain Res 77: 234–244.
Carlson SS, Hockfield S (1996) Central nervous system. In: Comper WD,ed. Extracellular Matrix Vol.1.Tissue Function. Amsterdam: Harwood Academic Publishers, pp. 1–23.
Celio MR (1993) Perineuronal nets of extracellular matrix around parvalbumin-containing neurons of the hippocampus. Hippocampus 3: 55–60.
Celio MR, Bl¨umcke I (1994) Perineuronal nets – a specialized form of extracellular matrix in the adult nervous system. Brain Res Rev 19: 128–145.
Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L (1998) Perineuronal nets: Past and present. Trends Neurosci 21: 510–515.
Chen L, Folsom DB, Ko CP (1991) The remodeling of synaptic extracellular matrix and its dynamic relationship with nerve terminals at living frog neuromuscular junctions. J Neurosci 11: 2920–2930.
Collin C, Miyaguchi K, Segal M (1997) Dendritic spine density and LTP induction in cultured hippocampal slices. J Neurophysiol 77: 1614–1623.
Dailey ME, Buchanan J, Bergles DE, Smith SJ (1994) Mossy fiber growth and synaptogenesis in rat hippocampal slices in vitro. J Neurosci 14: 1060–1078.
Debanne D, Gu´erineau NC, G¨ahwiler BH, Thompson SM (1995) Physiology and pharmacology of unitary synaptic connections between pairs of cells in areas CA3 and CA1 of rat hippocampal slice cultures. J Neurophysiol 73: 1282–1294.
De Jong BM, Ruijter JM, Romijn HJ (1988) Cytoarchitecture in cultured rat neocortex explants. Int J Dev Neurosci 6: 327–339.
Del Rio JA, Heimrich B, Soriano E, Schwegler H, Frotscher M (1991) Proliferation and differentiation of glial fibrillary acidic protein-immunoreactive glial cells in organotypic cultures of rat hippocampus. Neuroscience 43: 335–347.
Deyst KA, Toole BP (1995) Production of hyaluronan-dependent pericellular matrix by embryonic rat glial cells. Dev Brain Res 88: 122–125.
Drake CT, Mulligan KA, Wimpey TL, Hendrickson A, Chavkin C (1991) Characterization of Vicia villosa agglutinin-labeled GABAergic interneurons in the hippocampal formation and in acutely dissociated hippocampus. Brain Res 554: 176–185.
Fields RD, Nelson PG (1992) Activity-dependent development of the vertebrate nervous system. Int Rev Neurobiol 34: 133–214.
Frotscher M, Heimrich B (1995) Lamina-specific synaptic connections of hippocampal neurons in vitro. J Neurobiol 26: 350–359.
Frotscher M, Zafirov S, Heimrich B (1995) Development of identified neuronal types and of specific synaptic connections in slice cultures of rat hippocampus. Prog Neurobiol 45: 143–164.
G¨ahwiler BH (1981) Organotypic monolayer cultures of nervous tissue. J Neurosci Methods 4: 329–342.
G¨ahwiler BH (1984) Development of the hippocampus in vitro: Cell types, synapses and receptors. Neuroscience 11: 751–760.
G¨ahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM (1997) Organotypic slice cultures: Atechnique has come of age. Trends Neurosci 20: 471–477.
G¨otz M, Bolz J (1992) Formation and preservation of cortical layers in slice cultures. J Neurobiol 23: 783–802.
Guimaraes A, Zaremba S, Hockfield S (1990) Molecular and morpho-logical changes in the cat lateral geniculate nucleus and visual cortex induced by visual deprivation are revealed by monoclonal antibodies Cat-304 and-301. J Neurosci 10: 3014–3024.
H¨artig W, Brauer K, Bigl V, Br¨uckner G (1994) Chondroitin sulfateproteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res 635: 307–311.
H¨artig W, Brauer K, Br¨uckner G (1992) Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuro Report 3: 869–872.
H¨artig W, Derouiche A, Welt K, Brauer K, Grosche J, M¨ader M, Reichenbach A, Br¨uckner G (1999) Cortical neurons immuno-reactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res 842: 15–29
Hendry SHC, Jones EG, Hockfield S, McKay RDG (1988) Neuronal populations stained with the monoclonal antibody Cat-301 in the mammalian cerebral cortex and thalamus. J Neurosci 8: 518–542.
Hockfield S, Kalb RG, Zaremba S, Fryer H (1990) Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harbor Symp Quant Biol 55: 505–514.
Hockfield S, McKay RDG(1983) Asurface antigen expressed by a subset of neurons in the vertebrate central nervous system. Proc Natl Acad Sci USA 80: 5758–5761.
Hobohm C, H¨artig W, Brauer K, Br¨uckner G (1998) Low expression of extracellular matrix components in rat brain stem regions containing modulatory aminergic neurons. J Chem Neuroanat 15: 135–142.
Katz LC, Shatz CJ (1996) Synaptic activity and the construction of cortical circuits. Science 274: 1133–1138.
Kelly SS, Anis N, Robbins N(1985) Fluorescent staining of living mouse neuromuscular junctions. Pfl¨ugers Archiv 404: 97–99.
Kind PC, Beaver CJ, Mitchell DE (1995) Effects of early periods of monocular deprivation and reverse lid suture on the development of Cat-301 immunoreactivity in the dorsal lateral geniculate nucleus (dLGN) of the cat. J Comp Neurol 359: 523–536.
Klostermann O, Wahle P (1999) Patterns of spontaneous actvity and morphology of interneuron types in organotypic cortex and thalamus-cortex cultures. Neuroscience 9: 1243–1259.
Ko C-P (1987) Alectin, peanut agglutinin, as a probe for the extracellular matrix in living neuromuscular junctions. J Neurocytol 16: 567–576.
Ko C-P, Chen L (1996) Synaptic remodeling revealed by repeated in vivo observation and electron microscopy of identified frog neuromuscular junctions. J Neurosci 16: 1780–1790.
K¨oppe G, Br¨uckner G, H¨artig W, Brauer K, Bigl V (1997a) Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res 288: 33–41.
K¨oppe G, Br¨uckner G, H¨artig W, Delpech B, Bigl V (1997b) Characterization of proteoglycan-containing perineuronal nets by enzymatic treatments of rat brain sections. Histochem J 29: 11–20.
Kosaka T, Heizmann CW (1989) Selective staining of a population of parvalbumin-containing GABAergic neurons in the rat cerebral cortex by lectins with specific affinity for terminal N-acetylgalactosamine. Brain Res 483: 158–163.
Lander C, Kind P, Maleski M, Hockfield S (1997) A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex. J Neurosci 17: 1928–1939.
Lander C, Zhang H, Hockfield S (1998) Neurons produce a neuronal cell surface-associated chondroitin sulfate proteoglycan. J Neurosci 18: 174–183.
Leranth C, Szeidemann Z, Hsu M, Buzs´aki G (1996) AMPA receptors in the rat and primate hippocampus: A possible absence of GluR2/3 subunits in most interneurons. Neuroscience 70: 631–652.
Liu SJ, Kaczmarek K (1998) Depolarization selectively increases the expression of the Kv3.1 potassium channel in developing inferior colliculus neurons. J Neurosci 18: 8758–8769.
Lohmann C, Ilic V, Friauf E (1998) Development of a topographically organized auditory network in slice culture is calcium dependent. J Neurobiol 34: 97–112.
Maleski M, Hockfield S (1997) Glial cells assemble hyaluronan-based pericellular matrices in vitro. Glia 20: 193–202.
Martin LJ, Blackstone CD, Levey AI, Huganir RL, Price DL (1993) AMPA glutamate receptor subunits are differentially distributed in the rat brain. Neuroscience 53: 327–358.
Matsui F, Nishizuka M, Oohira A (1999) Proteoglycans in perineuronal nets. Acta Histochem Cytochem 32: 141–147.
Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S (2002) Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci 22: 7536–7547.
McGuire PK, Hockfield S, Goldman-Rakic PS (1989) Distribution of Cat-301 immunoreactivity in the frontal and parietal lobes of the macaque monkey. J Comp Neurol 288: 280–296.
Muller D, Buchs P-A, Stoppini L (1993) Time course of synaptic development in hippocampal organotypic cultures. Dev Brain Res 71: 93–100.
Murakami T, Murakami T, Hong LJ, Su WD, Piao DX, Mahmut N, Ohtsuka A(1997) Perineuronal sulfated proteoglycans and cell surface glycoproteins in adult and newborn mouse brains, with special reference to their postnatal developments. Arch Histol Cytol 60: 347–354.
Nakagawa F, Schulte BA, Wu JY, Spicer SS (1987) Postnatal appearance of glycoconjugate with terminal N-acetylgalactosamine on the surface of selected neurons in mouse brain. Dev Neurosci 9: 53–60.
Ohyama J, Ojima H (1997) Labeling of pyramidal and nonpyramidal neurons with lectin Vicia villosa during postnatal development of guinea pig. J Comp Neurol 389: 453–468.
Okamoto M, Sakiyama J, Kurazono S, Mori S, Nakata Y, Nakaya N, Oohira A (2001) Developmentally regulated expression of brain-specific chondroitin sulfate proteoglycans, neurocan and phosphacan, in the postnatal rat hippocampus. Cell Tissue Res 306: 217–229.
Ong WY, He Y, Tan KK, Garey LJ (1998) Differential localisation of the metabotropic glutamate receptor mGluR1a and the ionotropic glutamate receptor GluR2/3 in neurons of the human cerebral cortex. Exp Brain Res 119: 367–374.
Oohira A, Matsui F, Tokita Y, Yamauchi S, Aono S (2000) Molecular interactions of neural chondroitin sulfate proteoglycans in the brain development. Arch Biochem Biophys 374: 24–34.
Pavlidis P, Madison DV (1999) Synaptic transmission in pair recordings from CA3 pyramidal cells in organotypic culture. J Neurophysiol 81: 2787–2797.
Pozzo Miller LD, Petrozzini JJ, Mahanty NH, Connor JA (1993) Optical imaging of cytosolic calcium, electrophysiology, and ultrastructure in pyramidal neurons of organotypic slice cultures from rat hippocampus. Neuroimage 1: 109–120.
Robain O, Barbin G, Billette de Villemeur TB, Jardin L, Jahchan T, Ben-Ari Y (1994) Development of mossy fiber synapses in hippocampal slice cultures. Dev Brain Res 80: 244–250.
Seeger G, Brauer K, H¨artig W, Br¨uckner G (1994) Mapping of perineuronal nets in the rat brain stained by colloidal iron hydroxide histochemistry and lectin cytochemistry. Neuroscience 58: 371–388.
Seil FJ, Kelly JM, Leiman AL(1974) Anatomical organization of cerebral neocortex in tissue culture. Exp Neurol 45: 435–450.
Stoppini L, Buchs P-A, Muller D(1991) Asimple method for organotypic cultures of nervous tissue. J Neurosci Methods 37: 173–182.
Tole S, Christian C, Grove EA (1997) Early specification and autonomous development of cortical fields in the mouse hippocampus. Development 124: 4959–4970.
Van Ooyen A (1994) Activity-dependent neural network development. Network Comp Neural Syst 5: 401–423.
Vogt Weisenhorn DM, Celio MR, Rickmann M (1998) The onset of parvalbumin-expression in interneurons of the rat parietal cortex depends upon extrinsic factor(s). Eur J Neurosci 10: 1027–1036.
Watanabe E, Fujita SC, Murakami F, Hayashi M, Matsumura M (1989) A monoclonal antibody identifies a novel epitope surrounding a subpopulation of the mammalian central neurons. Neuroscience 29: 645–657.
Wintergerst ES, Vogt Weisenhorn DM, Rathjen FG, Riederer BM, Lambert S, Celio MR (1996) Temporal and spatial appearance of the membrane cytoskeleton and perineuronal nets in the rat neocortex. Neurosci Lett 209: 173–176.
Wolburg H, Bolz J (1991) Ultrastructural organization of slice cultures from rat visual cortex. J Neurocytol 20: 552–563.
Yamaguchi Y (2000) Lecticans: Organizers of the brain extracellular matrix. Cell Mol Life Sci 57: 276–289.
Yamamoto N, Kurotani T, Toyama K(1989) Neural connections between the lateral geniculate nucleus and visual cortex in vitro. Science 245: 192–194.
Yamamoto N, Yamada K, Kurotani T, Toyama K (1992) Laminar specificity of extrinsic cortical connections studied in coculture preparations. Neuron 9: 217–228.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brückner, G., Kacza, J. & Grosche, J. Perineuronal Nets Characterized by Vital Labelling, Confocal and Electron Microscopy in Organotypic Slice Cultures of Rat Parietal Cortex and Hippocampus. Histochem J 35, 115–122 (2004). https://doi.org/10.1023/B:HIJO.0000023374.22298.50
Issue Date:
DOI: https://doi.org/10.1023/B:HIJO.0000023374.22298.50