Abstract
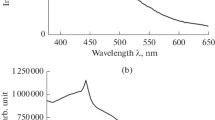
Europium(III) chloride is encapsulated into a porous glass through impregnation with a 0.1 M aqueous solution of the salt with subsequent elimination of water from the pore space. The results of comparing the intercalate content with spatially geometric parameters of the carrier, the increased adsorption of water by a modified glass, and the character of luminescence quenching due to the adsorption of water indicate that the salt particles in the porous glass have nanometer sizes.
Similar content being viewed by others
REFERENCES
Maas, H., Currao, A., and Calzaferri, G., Encapsulated Lanthanides as Luminescent Materials, Angew. Chem., 2002, vol. 41, no. 14, pp. 2495–2497.
Justel, T., Wiechert, D.U., Lau, C., Sendor, D., and Kynast, U., Optically Functional Zeolites: Evaluation of UV and VUV Stimulated Photoluminescence Properties of Ce3+-and Tb3+-Doped Zeolite X, Adv. Funct. Mater.,2001, vol. 11, no. 2, pp. 105–110.
Selvan, T.S., Hayakawa, T., and Nogami, M., Enhanced Fluorescence from Eu3+-Doped Silica Gels by Adsorbed CdS Nanoparticles, J. Non-Cryst. Solids, 2001, vol. 291, nos. 1-2, pp. 137–141.
Yang, P., Zhao, D., Margolese, D.I., Chmelka, B.F., and Stucky, G.D., Generalized Syntheses of Large-Pore Mesoporous Metal Oxides with Semicrystalline Frameworks, Nature (London), 1998, no. 396, pp. 152–155.
Nassar, E.J., Serraa, O.A., Calefia, P.S., Mansoa, S.M.C.P., and Neria, C.R., β-Diketonates of Eu3+ Red Phosphors Supported on Functionalized Silica, Mater. Res. Bull., 2001, vol. 4, no. 1, pp. 18–22.
Verezhinskaya, R.L., Burkat, T.M., Pak, V.N., and Rychgorskii, V.V., Distribution of Silver in a Porous Glass from the Data on Electrical Conductivity, Fiz. Khim. Stekla, 1999, vol. 25, no. 6, pp.688–692 [Glass Phys. Chem. (Engl. transl.), 1999, vol. 25, no. 6, pp. 519-522].
Pak, V.N. and Sukhanov, S.V., Optical Properties of Porous Glasses Modified by Vanadium(V) Oxide, Zh. Prikl. Khim. (St. Petersburg), 2003, vol. 76, no. 8, pp. 1241–1244.
Pak, V.N., Verezhinskaya, R.L., and Burkat, T.M., The Influence of Conditions of Reducing AgNO3 on the Distribution of Silver in a Porous Glass, Zh. Fiz. Khim., 2002, vol. 76, no. 7, pp. 1324–1327.
Tur, T.M., Metelkina, Yu.S., and Pak, V.N., Specific Features of Electrical Conductivity of Tungsten Oxide with Mixed Oxidation States on the Surface of γ-Al2O3, Zh. Prikl. Khim. (St. Petersburg), 2000, vol. 73, no. 1, pp.48–52.
Pak, V.N., Sukhanov, S.V., and Gavronskaya, Yu.Yu., Electrical Conductivity of Carbon in Porous Glasses, Zh. Prikl. Khim. (St. Petersburg), 2002, vol. 75, no. 10, pp. 1651–1654.
Molchanova, O.S., Natrievo-borosilikatnye i poristye stekla (Sodium Borosilicate and Porous Glasses), Moscow: Oborongizdat, 1961.
Roskova, G.P. and Tsekhomskaya, T.S., Use of Phase Separation Phenomena for Preparing Glasses and Materials with Specified Properties, Fiz. Khim. Stekla, 1981, vol. 7, no. 5, pp. 513–534.
Charlot, G., Les methodes de la chimie analytique: Analyse quantitative minerale, Paris: Masson, 1961, 4 ed. Translated under the title Metody analiticheskoi khimii, Moscow: Khimiya, 1969, part 2.
Andreeva, D.A. and Puzyk, M.V., Quenching of Luminescence of Cyclometallized Pt(II) Complexes by Molecular Oxygen, Opt. Spektrosk., 2003, vol. 95, no. 5, pp. 764–765.
Poluektov, N.S., Kononenko, L.I., Efryushina, N.P., and Bel'tyukova, S.V., Spektrofotometricheskie i lyuminestsentnye metody opredeleniya lantanoidov (Spectrophotometric and Luminescence Methods for Determining Lanthanides), Kiev: Naukova Dumka, 1989.
Stump, N.A., Schweitzer, G.K., Gubson, J.K., Haire, R.G., and Peterson, J.R., Luminescence Study of the Thermal Decomposition of Europium Trichloride Hexahydrate, EuCl3 6H2O, Appl. Spectrosc., 1994, vol. 48, no. 8, pp. 937–943.
Davidenko, N.K. and Lugina, N.L., Spectra and Structure of Aqua Ions of Neodymium, Europium, and Erbium, Zh. Neorg. Khim., 1968, vol. 13, no. 4, pp. 980–987.
Sorbenty na osnove silikagelya v radiokhimii(Sorbents Based on Silica in Radiochemistry), Laskorin, B.N., Ed., Moscow: Atomizdat, 1977.
Pak, V.N., Nikolaev, Yu.S., Ragulin, G.K., and Kharangi, S., Adsorption of Water Vapors by Surface Polysilicates of Copper, Nickel, and Cobalt, Zh. Fiz. Khim., 1996, vol. 70, no. 12, pp. 2256–2259.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Petushkov, A.A., Shilov, S.M., Puzyk, M.V. et al. Adsorption and Luminescence Properties of Porous Glasses Containing Europium(III) Chloride Nanoparticles. Glass Physics and Chemistry 30, 333–336 (2004). https://doi.org/10.1023/B:GPAC.0000038705.34391.4c
Issue Date:
DOI: https://doi.org/10.1023/B:GPAC.0000038705.34391.4c