Abstract
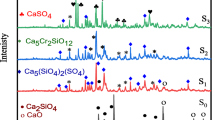
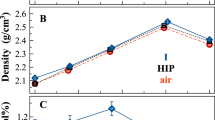
High-alkali silicate glasses M-27 containing chloride additives (MCl, where M = Na and K) introduced in the course of synthesis (0, 1, 5, 10, 12.5 wt % Cl) are investigated. The glasses synthesized are analyzed for chlorine content, and their thermal and electrical characteristics are measured. The upper limits of the chlorine content in the glasses are determined. The nonlinear dependence of the as-analyzed chlorine content in a binary glass on the as-batched chlorine content introduced into a batch is explained by structural transformations during which chlorine in the glass interacts with polar groupings that are bonded to at least two polar tetrahedra. Only large-sized associates are able to fix chlorine. The proposed concept of structural transformations is used to interpret the changes observed in the bulk (thermal, electrical) parameters of glasses. It is shown that these changes are governed by two competitive processes, namely, the incorporation of chlorine into the glass structure and the change in the total glass composition due to volatilization of the components in the course of the synthesis. It is noted that the effect of chloride additives on the high-temperature viscosity of glass-forming melts is weaker than that of fluoride additives.
Similar content being viewed by others
REFERENCES
Appen, A.A., Khimiya stekla (Chemistry of Glass), Leningrad: Khimiya, 1974.
Callow, R.J., The Solubility of Fluorides in Glass: Part I, J. Soc. Glass Technol., 1949, vol. 33, no. 153, pp. 255–266.
Bock, R., A Handbook of Decomposition Methods in Analytical Chemistry, Weinheim: Blackie, 1979. Translated under the title Metody razlozheniya v analiticheskoi khimii, Moscow: Khimiya, 1984.
Scoog, D.A. and West, D.M., Fundamentals of Analytical Chemistry, New York: Holt, Rinehart, and Winston, 1976. Translated under the title Osnovy analiticheskoi khimii, Moscow: Mir, 1979, vols. 1, 2.
Mazurin, O.V., Electrical Properties of Glasses, Tr. Leningr. Tekhnol. Inst. im. Lensoveta, 1962, no. 62.
Mazurin, O.V., Totesh, A.S., Strel'tsina, M.V., and Shvaiko-Shvaikovskaya, T.P., Teplovoe rasshirenie stekla (Thermal Expansion of Glasses), Leningrad: Nauka, 1969.
Kiprianov, A.A. and Karpukhina, N.G., Investigation into Properties of Oxohalide Electrode Glasses, in Mezhvuz. sb. “Ionnyi obmen i ionometriya” (Intercollege Collection of Articles “Ion Exchange and Potentiometry, St. Petersburg Gos. Univ., 1999, no. 10, pp. 178–196.
Hirayama, C. and Camp, F.T., The Effect of Fluorine and Chlorine Substitution on the Viscosity and Fining of Soda-Silicate and a Potassium-Barium Silicate Glass, Glass Technol., 1969, vol. 10, no. 5, pp. 123–127.
Mogileva, V.V., A Study of Concentration Distribution and Interdiffusion of Ions in Surface Layers of Sodium Aluminosilicate Glass Treated in Aqueous Solutions, Cand. Sci. Dissertation, Leningrad: Leningrad State Univ., 1978.
Kiprianov, A.A., Karpukhina, N.G., and Molodozhen, V.A., Investigation into the Influence of Fluorine on the Bulk and Electrode Properties of Lithium Silicate Glasses, Vestn. St. Petersburg Gos. Univ. Ser. 4: Fiz., Khim., 1998, issue 3, no. 18, pp. 53–58.
Bezborodov, M.A., Sintez i stroenie silikatnykh stekol (Synthesis and Structure of Silicate Glasses), Minsk: Nauka i Tekhnika, 1968.
Ryabchikov, I.D., Experimental Investigation into the Distribution of Alkali Elements among Immiscible Silicate and Chloride Melts, Dokl. Akad. Nauk SSSR, 1963, vol. 149, no. 5, pp. 1174–1177.
Bragina, G.I. and Anfilov, V.N., Phase Relationships in the Na2O-NaF-SiO2 and Na2O-SiO2-NaCl Glass-Forming Systems, Fiz. Khim. Stekla, 1977, vol. 3, no. 5, pp. 476–479.
Shumilin, A.N., Issledovanie roli khloristogo natriya kak dobavki i uskoritelya varki vysokoglinozemistykh i sodovo-izvestkovykh silikatnykh stekol (Investigation of Role of Sodium Chloride as Additive and Promoter of Melting of High-Alumina and Soda-Lime Glasses), Minsk: BPI, 1957.
Stevels, J.M., The Electrical Properties of Glass, Berlin: Springer-Verlag, 1957. Translated under the title Elektricheskie svoistva stekla, Moscow: Inostrannaya Literatura, 1961.
Mazurin, O.V., Strel'tsina, M.V., and Shvaiko-Shvaikovskaya, T.P., Svoistva stekol i stekloobraznykh rasplavov (Properties of Glasses and Glass-Forming Melts), Leningrad: Nauka, 1973, vols. 1-3.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kiprianov, A.A., Karpukhina, N.G. & Molodozhen, V.A. Investigation into the Influence of Chloride Additives on the Properties of Alkali Silicate Glasses. Glass Physics and Chemistry 30, 325–332 (2004). https://doi.org/10.1023/B:GPAC.0000038704.68182.72
Issue Date:
DOI: https://doi.org/10.1023/B:GPAC.0000038704.68182.72