Abstract
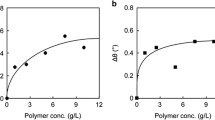
Carbohydrate-carbohydrate interactions between clustered GM3 on the Langmuir monolayer and clustered Gg3 trisaccharide along a polystyrene chain were investigated using surface pressure-area (π-A) isotherms and surface plasmon resonance (SPR). The π-A isotherm of the GM3 monolayer was expanded substantially and specifically by the Gg3-trisaccharide-bearing glycoconjugate polystyrene [PN(Gg3)] even at 10−12 M. The PN(Gg3)-induced expansion of the GM3 monolayer required no calcium ion, and the expansion was strongly inhibited in the presence of urea and acetamido sugars. SPR studies of the GM3-Gg3 interaction were carried out to estimate the affinity constant and specificity of the interaction quantitatively. PN(Gg3) was adsorbed onto the GM3 monolayer strongly and specifically with an apparent affinity constant of K a = 2.5 × 106 M−1. The mechanism of the GM3-Gg3 interaction was discussed on the basis of the relationship between affinity and structure. We found that the NHAc groups of N-acetylneuraminic acid in GM3 and of GalNAc in Gg3 play an important role in the GM3-Gg3 interaction and that PN(Gg3) recognizes not only some specified portions of GM3 but also the trisaccharide as a whole. Published in 2004.
Similar content being viewed by others
References
Eggenst I, Fenderson B, Toyokuni T, Dean B, Stroud M, Hakomori S, Specific interaction between Le X and Le X determinants, J Biol Chem 264, 9476-84 (1989).
Hakomori S, Carbohydrate-carbohydrate interaction as an initial step in cell recognition, Pure Appl Chem 63, 473-82 1991
Iwabuchi K, Yamamura S, Prinetti A, Handa K, Hakomori S, GM3-enriched microdomain involved in cell adhesion and signal trans-duction through carbohydrate-carbohydrate interaction in mouse melanoma B16 cells, J Biol Chem 273, 9130-8 (1998).
Song Y, Withers DA, Hakomori S, Globoside-dependent adhe-sion of human embryonal carcinoma cells, based on carbohydrate-carbohydrate interaction, initiates signal transduction and induces enhanced activity of transcription factors AP1 and CREB, J Biol Chem 273, 2517-25 (1998).
Stewart RJ, Boggs JM, A carbohydrate-carbohydrate interaction between galactosylceramide-containing liposomes and cerebro-side sulfate-containing liposomes: Dependence on the glycolipid ceramide composition, Biochemistry 32, 10666-74 (1993).
Koshy KM, Boggs JM, Investigation of the calcium-mediated as-sociation between the carbohydrate head groups of galactosyl-ceramide and galactosylceramide I 3 sulfate by electrospray ionization mass spectrometry, J Biol Chem 271, 3496-9 (1996).
Koshy KM, Wang J, Boggs JM, Divalent cation-mediated interac-tion between cerebroside sulfate and cerebrosides: An investigation of the effect of structural variations of lipids by electrospray ionization mass spectrometry, Biophys J 77, 306-18 (1999).
Boggs JM, Menikh A, Rangaraj G, Trans interactions between galactosylceramide and cerebroside sulfate across apposed bilayers, Biophys J 78, 874-85 (2000).
Lemieux RU, Howwater provides the impetus for molecular recog-nition in aqueous solution, Acc Chem Res 29, 373-80 (1996).
Lis H, Sharon N, Lectins: Carbohydrate-specific proteins that me-diate cellular recognition, Chem Rev 98, 637-74 (1998).
Dam TK, Brewer CF, Thermodynamic studies of lectin-carbohydrate interactions by isothermal titration calorimetry, Chem Rev 102, 387-429 (2002).
Wormald MR, Edge CJ, DwekRA, The solution coformation of the Le X group, Biochem Biophys Res Commun 180, 1214-21 (1991).
Geyer A, Gege C, Schmidt RR, Carbohydrate-carbohydrate recog-nition between Lewis X glycoconjugates, Angew Chem Int Ed 38,1466-8 (1999).
Simons K, Ikonen E, Functional rafts in cell membranes, Nature 387, 569-72 (1997).
Simons K, Toomre D, Lipid rafts and signal transduction, Nat Rev Mol Cell Biol 1, 31-9 (2000).
Lee YC, Biochemistry of carbohydrate-protein interaction, FASEB J 6, 3193-200 (1992).
Kiessling LL, Pohl NL, Strength in numbers: Non-natural polyva-lent carbohydrate derivatives, Chem Biol 3, 71-7 (1996).
Roy R, Blue-prints, synthesis and applications of glycopolymers, Trends Glycosci Glycotech 8, 79-99 (1996).
Mammen M, Choi S-K, Whitesides GM, Polyvalent interactions in biological systems: Implications for design and use of multi-valent ligands and inhibitors, Angew Chem Int Ed 37, 2754-94 (1998).
Lundquist JJ, Toone EJ, The cluster glycoside effect, Chem Rev 102, 555-78 (2002).
Bovin NV, Polyacrylamide-based glycoconjugates as tools in gly-cobiology, Glycoconjugate J 15, 431-46 (1998).
Kobayashi K, Tsuchida A, Usui T, Akaike T, A new type of ar-tificial glycoconjugate polymer: A convenient synthesis and its interaction with lectins, Macromolecules 30, 2016-20 (1997).
Sigal GB, Mammem M, Dahmann G, Whitesides GM, Polyacryl-amides bearing pendant á-sialoside groups strongly inhibit agglu-tination of erythrocytes by influenza virus: The strong inhibition reflects enhanced binding through cooperative polyvalent interac-tions, J AmChem Soc 118, 3789-800 (1996).
Manning DD, Hu X, Kiessling LL, Synthesis of sulfated neogly-copolymers: Selective P-selectin inhibitors, J AmChem Soc 119, 3161-2 (1997).
Kamitakahara H, Suzuki T, Nishigori N, Suzuki Y, Kanie O, Wong CH,Alysoganglioside/poly-L-glutamic acid conjugate as a pico-molar inhibitor of influenza hemagglutinin,Angew Chem Int Ed 37, 1524-8 (1998).
Sasaki K, Nishida Y, Tsurumi T, Uzawa H, Kondo H, Kobayashi K, Facile assembly of cell surface oligosaccharide mimics by copolymerization of carbohydrate modules, Angew Chem Int Ed 41, 4463-7 (2002).
Aoi K, Itoh K, Okada M, Globular carbohydrate macro-molecule "sugar balls". 1. synthesis of novel sugar-persubstituted poly(amido amine)dendrimers, Macromolecules 28, 5391-3 (1995).
Aoi K, Itoh K, Okada M, Divergent/convergent joint approach with a half-protected initiator core to synthesize surface-block den-drimers, Macromolecules 30, 8072-4 (1997).
Zanini D, Roy R, Synthesis of new-thiosialodendrimers and their binding properties to the sialic acid specific lectin from Limax flavus, J AmChem Soc 119, 2088-95 (1997).
Hansen HC, Haataja S, Finne J, Magnusson G, Di-, tri-, and tetrava-lent dendritic galabiosides that inhibit hemagglutination by Strep-tococcus suisat nanomolar concentration, J AmChem Soc 119, 6974-9 (1997).
Matsuura K, Akasaka T, Hibino M, Kobayashi K, Facile synthesis of stable and lectin-recognizable DNA-carbohydrate conjugates via diazo coupling, Bioconjugate Chem 11, 202-11 (2000).
Matsuura K, Hayashi K, Kobayashi K, Artificial regulation of tran-scription applying carbohydrate-lectin interaction, Chem Com-mun 1140-1 (2002).
Akasaka T, Matsuura K, Kobayashi K, Transformation from block-type to graft-type oligonucleotide-glycopolymer conjugates by self-organization with half-sliding complementary oligonu-cleotides and their lectin recognition, Bioconjugate Chem 12, 776-85 (2001).
Matsuura K, Hibino M, Yamada Y, Kobayashi K, Construction of glyco-clusters by self-organization of site-specifically glyco-sylated oligonucleotides and their cooperative amplification of lectin-recognition, J AmChem Soc 123, 357-8 (2001).
Matsuura K, Hibino M, Ikeda T, Yamada Y, Kobayashi K, Self-organized glycoclusters along DNA: Effect of the spatial arrange-ment of galactoside residues on cooperative lectin recognition, Chem Eur J 10, 352-9 (2004).
Wang Y, Sheppard TL, Chemoenzymatic synthesis and antibody detection of DNA glycoconjugates, Bioconjugate Chem 14, 1314-22 (2003).
Sakai S, Sasaki T, Multivalent carbohydrate ligands assembled on a metal template, J AmChem Soc 116, 1587-8 (1994).
Hasegawa T, Yonemura T, Matsuura K, Kobayashi K, Artificial metalloglycoclusters: Compact saccharide shell to induce high lectin affinity as well as strong luminescence, Biocojugate Chem 14, 728-37 (2003).
Ariga K, Kunitake T, Molecular recognition at air-water and re-lated interfaces: Complementary hydrogen bonding and multisite interaction, Acc Chem Res 31, 371-8 (1998)
Okahata Y, Ebara Y, A Kinetic Study of Concanavalin A binding to glycolipid monolayers by using a quartz-crystal microbalance, J AmChem Soc 116, 11209-12 (1994).
Sato T, Serizawa T, Okahata Y, Binding of influenza A virus to monosialoganglioside (GM3) reconstituted in glucosylceramide and sphingomyelin membranes, Biochem Biophys Acta 1285, 14-20 (1996).
Sato T, Serizawa T, Otake F, Nakamura M, Terabayashi T, Kawanishi Y, Okahata Y, Quantitative measurements of the inter-action between/monosialoganglioside monolayers and wheat germ agglutinin WGA by a quartz-crystal microbalance, Biochem Bio-phys Acta 1380, 82-92 (1998).
Mann DA, Kanai M, Maly DJ, Kiessling LL, Probing low affinity and multivalent interactions with surface plasmon resonance: Lig-ands for concanavalin A, J AmChem Soc 120, 10575-82 (1998).
MacKanzie CR, Hirama T, Lee KK, Altman E, Young NM, Quan-titative analysis of bacterial toxin affinity and specificity for gly-colipid receptors by surface plasmon resonance, J Biol Chem 272, 5533-8 (1997).
Matsuura K, Kitakouji H, Tsuchida A, Sawada N, Ishida H, Kiso M, Kobayashi K, Carbohydrate-carbohydrate interaction between glycolipids and glycoconjugate polystyrenes at the air-water inter-face, Chem Lett 1293-4 (1998).
Matsuura K, Kitakouji H, Oda R, Morimoto Y, Asano H, Ishida H, Kiso M, Kitajima K, Kobayashi K, Selective expansion of the GM3 glycolipid monolayer induced by carbohydrate-carbohydrate interaction with Gg3 trisaccharide-bearing glyco-conjugate polystyrene at the air-water interface, Langmuir 18, 6940-5 (2002).
Matsuura K, Kitakouji H, Sawada N, Ishida H, Kiso M, Kitajima K, Kobayashi K, A quantitative estimation of carbohydrate-carbohydrate interaction using clustered oligosac-charides of glycolipid monolayers and of artificial glycoconjugate polymers by surface plasmon resonance, J AmChem Soc 122, 7406-7 (2000).
Matsuura K, Oda R, Kitakouji H, Kiso M, Kitajima K, Kobayashi K, Surface plasmon resonance study of carbohydrate-carbohydrate interaction between various gangliosides and Gg3-carrying polystyrene, Biomacromolecules 5, 937-41 (2004).
Wataoka I, Urakawa H, Kobayashi K, Akaike T, Kajiwara K, Structural characterization of glycoconjugate polystyrene in aqueous solution, Macromolecules 32, 1816-21 (1999).
Ohno K, Tsujii Y, Miyamoto T, Fukuda T, Goto M, Kobayashi K, Akaike T, Synthesis of a well-defined glycopolymer by nitroxide-controlled free radical polymerization, Macromolecules 31, 1064-9 (1998).
Matsuura K, Tsuchida A, Okahata Y, Akaike T, Kobayashi K, A quartz-crystal microbalance study of adsorption behaviors of artificial glycoconjugate polymers onto chemically modified gold surfaces and their interactions with lectins, Bull Chem Soc Jpn 71, 2973-7 (1998).
Tsuchida A, Matsuura K, Kobayashi K, Aquartz-crystal microbal-ance study of adsorption behaviors of artificial glycoconjugate polymers with different saccharide chain lengths and with dif-ferent backbone structures,Macromol Chem Phys 201, 2245-50 (2000).
Maggio B, The surface behavior of glycosphingolipids in biomem-branes: A new frontier of molecular ecology, Prog Biophys Mol Biol 62, 55-117 (1994).
Yu S, Kojima N, Hakomori S, Kudo S, Inoue S, Inoue Y, Binding of rainbow trout sperm to egg is mediated by strong carbohydrate-to-carbohydrate interaction between (KDN)GM3 (deaminated neu-raminylganglioside) and Gg3-like epitope, Proc Natl Acad Sci USA 99, 2854-9 (2002).
Santacroce PV, Basu A, Probing specificity in carbohydrate-carbohydrate interactions with micelles and Langmuir monolay-ers, Angew Chem Int Ed 42, 95-8 (2003).
Hernáiz MJ, de la Fuente JM, Barrientos AG, Penadés S, A model system mimicking glycosphingolipid clusters to quantify carbohy-drate self-interactions by surface plasmon resonance, AngewChem Int Ed 41, 1554-7 (2002).
Yu ZW, Calvert TL, Leckband D, Molecular forces between mem-branes displaying neutral glycosphingolipids: Evidence for carbo-hydrate attraction, Biochemistry 37, 1540-50 (1998).
Tromas C, Rojo J, de la Fuente JM, Barrientos AG, García R, Penadés S, Adhesion forces between Lewis X determinant antigens as measured by atomic force microscopy, Angew Chem Int Ed 40, 3052-5 (2001).
Rights and permissions
About this article
Cite this article
Matsuura, K., Kobayashi, K. Analysis of GM3-Gg3 interaction using clustered gycoconjugate models constructed from glycolipid monolayers and artificial glycoconjugate polymers. Glycoconj J 21, 139–148 (2004). https://doi.org/10.1023/B:GLYC.0000044845.64354.a3
Issue Date:
DOI: https://doi.org/10.1023/B:GLYC.0000044845.64354.a3