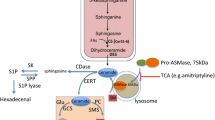
Abstract
Sphingolipids have been implicated in various cellular processes including growth, cell-cell or ligand-receptor interactions, and differentiation. In addition to their importance as reservoirs of metabolites with important signaling properties, sphingolipids also help provide structural order to plasma membrane lipids and proteins within the bilayer. Glycosylated sphingolipids, and sphingomyelin in particular, are involved in the formation of lipid rafts. Although it is well accepted that ceramide, the backbone of all sphingolipids, plays a critical role in apoptosis, less is known about the biological functions of glycosphingolipids. This review summarizes current knowledge of the involvement of glycosphingolipids in cell death and in other pathological processes and diseases. Published in 2004.
Similar content being viewed by others
References
Hakomori S, Glycosylation defining cancer malignancy: New wine in an old bottle, Proc Natl Acad SciUSA 99, 10231–3 (2002).
Lingwood CA, Oligosaccharide receptors for bacteria: A view to a kill, Curr Opin Chem Biol 2, 695–700 (1998).
Hirabayashi Y, Hamaoka A, Matsumoto M, Matsubara T, Tagawa M, Wakabayashi S, Taniguchi M, Syngeneic monoclonal antibody against melanoma antigen with interspecies cross-reactivity recognizes GM3, a prominent ganglioside of B16 melanoma, J Biol Chem 260, 13328–33 (1985).
Nores GA, DohiB T, Taniguchi M, Hakomori S, Density-dependent recognition of cell surface GM3 by a certain anti-melanoma antibody, and GM3 lactone as a possible immunogen: Requirements for tumor-associated antigen and immunogen, J Immunol 139, 3171–6 (1987).
Kojima N, Shiota M, Sadahira Y, Handa K, Hakomori S, Cell adhesion in a dynamic flow system as compared to static system. Glycosphingolipid-glycosphingolipid interaction in the dynamic system predominates over lectin-or integrin-based mechanisms in adhesion of B16 melanoma cells to non-activated endothelial cells, J Biol Chem 267, 17264–70 (1992).
Liu YY, Han TY, Giuliano AE, Ichikawa S, Hirabayashi Y, Cabot MC, Glycosylation of ceramide potentiates cellular resistance to tumor necrosis factor-alpha-induced apoptosis, Exp Cell Res 252, 464–70 (1999).
Liu YY, Han TY, Giuliano AE, Cabot MC, Ceramide glycosylation potentiates cellular multidrug resistance, FASEB J 15, 719–30 (2001).
Morjani H, Aouali N, Belhoussine R, Veldman RJ, Levade T, Manfait M, Elevation of glucosylceramide in multidrug-resistant cancer cells and accumulation in cytoplasmic droplets, Int J Cancer 94, 157–65 (2001).
Hakomori SI, Cell adhesion/recognition and signal transduction through glycosphingolipid microdomain, Glycoconj J 17, 143–51 (2000).
Kurzchalia TV, Parton RG, Membrane microdomains and caveolae, Curr Opin Cell Biol 11, 424–31 (1999).
van Blitterswijk WJ, van der Luit AH, Caan W, Verheij M, Borst J, Sphingolipids related to apoptosis from the point of view of membrane structure and topology, Biochem Soc Trans 29, 819–24 (2001).
van Meer G, Lisman Q, Sphingolipid transport: Rafts and translocators, J Biol Chem 277, 25855–8 (2002).
Anderson RG, Jacobson K, A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains, Science 296, 1821–5 (2002).
Hinkovska-Galcheva VT, Boxer LA, Mansfield PJ, Harsh D, Blackwood A, Shayman JA, The formation of ceramide-1-phosphate during neutrophil phagocytosis and its role in liposome fusion, J Biol Chem 273, 33203–9 (1998).
Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S, Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate, Nature 381, 800–3 (1996).
Spiegel S, Milstien S, Sphingosine-1-phosphate: Signaling inside and out, FEBS Lett 476, 55–7 (2000).
Hannun YA, Bell RM, Functions of sphingolipids and sphingolipid breakdown products in cellular regulation, Science 243, 500–7 (1989).
Hannun YA, Luberto C, Argraves KM, Enzymes of sphingolipid metabolism: From modular to integrative signaling, Biochemistry 40, 4893–903 (2001).
Snell EE, Dimari SJ, Brady RN, Biosynthesis of sphingosine and dihydrosphingosine by cell-free systems from Hansenula ciferri, Chem Phys Lipids 5, 116–38 (1970).
Weiss B, Stoffel W, Human and murine serine-palmitoyl-CoA transferase—cloning, expression and characterization of the key enzyme in sphingolipid synthesis, Eur J Biochem 249, 239–47 (1997).
Trinchera M, Ghidoni R, Sonnino S, Tettamanti G, Recycling of glucosylceramide and sphingosine for the biosynthesis of gangliosides and sphingomyelin in rat liver, Biochem J 270, 815–20 (1990).
Michel C, van Echten-Deckert G, Rother J, Sandhoff K, Wang E, Merrill AH, Jr, Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide, J Biol Chem 272, 22432–7 (1997).
Mandon EC, Ehses I, Rother J, van Echten G, Sandhoff K, Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase, and sphinganine N-acyltransferase in mouse liver, J Biol Chem 267, 11144–8 (1992).
Michel C, van Echten-Deckert G, Conversion of dihydroceramide to ceramide occurs at the cytosolic face of the endoplasmic reticulum, FEBS Lett 416, 153–5 (1997).
Albi E, Magni MV, Sphingomyelin synthase in rat liver nuclear membrane and chromatin, FEBS Lett 460, 369–72 (1999).
Futerman AH, Stieger B, Hubbard AL, Pagano RE, Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus, J Biol Chem 265, 8650–7 (1990).
Miro Obradors MJ, Sillence D, Howitt S, Allan D, The subcellular sites of sphingomyelin synthesis in BHK cells, Biochim Biophys Acta 1359, 1–12 (1997).
Basu S, Kaufman B, Roseman S, Enzymatic synthesis of glucocerebroside by a glucosyltransferase from embryonic chicken brain, J Biol Chem 248, 1388–94 (1973).
Basu S, Schultz AM, Basu M, Roseman S, Enzymatic synthesis of galactocerebroside by a galactosyltransferase from embryonic chicken brain, J Biol Chem 246, 4272–9 (1971).
Keenan TW, Morre DJ, Basu S, Ganglioside biosynthesis. Concentration of glycosphingolipid glycosyltransferases in Golgi apparatus from rat liver, J Biol Chem 249, 310–5 (1974).
Basu SC, The serendipity of ganglioside biosynthesis: Pathway to CARS and HY-CARS glycosyltransferases, Glycobiology 1, 469–75 (1991).
Basu S, Basu M, Dastgheib S, Hawes J, Biosynthesis and regulation of glycosphingolipids. In Comprehensive Natural Product Chemistry edited by Barton D, Nakanishi K, Meth-Cohen O, Vol. 3 (ed. Pinto BM) (Pergamon Press, New York, 1999) pp. 107–28.
Yamashita T, Wada R, Sasaki T, Deng C, Bierfreund U, Sandhoff K, Proia RL, A vital role for glycosphingolipid synthesis during development and differentiation, Proc Natl Acad Sci USA 96, 9142–7 (1999).
Futerman AH, Pagano RE, Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver, Biochem J 280, 295–302 (1991).
Jeckel D, Karrenbauer A, Burger KN, van Meer G, Wieland F, Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions, J Cell Biol 117, 259–67 (1992).
Warnock DE, Lutz MS, Blackburn WA, Young WW Jr, Baenziger JU, Transport of newly synthesized glucosylceramide to the plasma membrane by a non-Golgi pathway, Proc Natl Acad Sci USA 91, 2708–12 (1994).
Coetzee T, Suzuki K, Popko B, New perspectives on the function of myelin galactolipids, Trends Neurosci 21, 126–30 (1998).
Kolter T, Sandhoff K, Sphingolipids—Their metabolic pathways and the pathobiochemistry of neurodegenerative diseases, Angew Chem Int Ed 38, 1532–68 (1999).
Schulte S, Stoffel W, Ceramide UDPgalactosyltransferase from myelinating rat brain: Purification, cloning, and expression, Proc Natl Acad Sci USA 90, 10265–9 (1993).
gFunato K, Riezman H, Vesicular and nonvesicular transport of ceramide from ER to the Golgi apparatus in yeast, J Cell Biol 155, 949–59 (2001).
Kok JW, Babia T, Klappe K, Egea G, Hoekstra D, Ceramide transport from endoplasmic reticulum to Golgi apparatus is not vesicle-mediated, Biochem J 333, 779–86 (1998).
van Meer G, Holthuis JC, Sphingolipid transport in eukaryotic cells, Biochim Biophys Acta 1486, 145–70 (2000).
Lannert H, Bunning C, Jeckel D, Wieland FT, Lactosylceramide is synthesized in the lumen of the Golgi apparatus, FEBS Lett 342, 91–6 (1994).
Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR, Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography, Proc Natl Acad Sci USA 98, 2399–406 (2001).
Hannun YA, Obeid LM, The ceramide-centric universe of lipidmediated cell regulation: Stress encounters of the lipid kind, J Biol Chem 277, 25847–50 (2002).
Kolesnick R, Hannun YA, Ceramide and apoptosis, Trends Biochem Sci 24, 224–5; discussion 227 (1999).
Birbes H, El Bawab S, Hannun YA, Obeid LM, Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis, Faseb J 15, 2669–79 (2001).
De Maria R, Lenti L, Malisan F, d'Agostino F, Tomassini B, Zeuner A, Rippo MR, Testi R, Requirement for GD3 ganglioside in CD95-and ceramide-induced apoptosis, Science 277, 1652–5 (1997).
Bhunia AK, Schwarzmann G, Chatterjee S, GD3 recruits reactive oxygen species to induce cell proliferation and apoptosis in human aortic smooth muscle cells, J Biol Chem 277, 16396–402 (2002).
Garcia-Ruiz C, Colell A, Paris R, Fernandez-Checa JC, Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation, FASEB J 14, 847–58 (2000).
Rippo MR, Malisan F, Ravagnan L, Tomassini B, Condo I, Costantini P, Susin SA, Rufini A, Todaro M, Kroemer G, Testi R, GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion, Faseb J 14, 2047–54 (2000).
Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC, Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione, J Biol Chem 272, 11369–77 (1997).
Kristal BS, Brown AM, Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition, J Biol Chem 274, 23169–75 (1999).
Scorrano L, Petronilli V, Di Lisa F, Bernardi P, Commitment to apoptosis by GD3 ganglioside depends on opening of the mitochondrial permeability transition pore, J Biol Chem 274, 22581–5 (1999).
Green DR, Reed JC, Mitochondria and apoptosis, Science 281, 1309–12 (1998).
Jacobson MD, Reactive oxygen species and programmed cell death, Trends Biochem Sci 21, 83–6 (1996).
Simon BM, Malisan F, Testi R, Nicotera P, Leist M, Disialoganglioside GD3 is released by microglia and induces oligodendrocyte apoptosis, Cell Death Differ 9, 758–67 (2002).
Ferri KF, Kroemer G, Organelle-specific initiation of cell death pathways, Nat Cell Biol 3, E255–63 (2001).
Tsujimoto Y, Shimizu S, The voltage-dependent anion channel: An essential player in apoptosis, Biochimie 84, 187–93 (2002).
Harris MH, Thompson CB, The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability, Cell Death Differ 7, 1182–91 (2000).
Liu Y, Fiskum G, Schubert D, Generation of reactive oxygen species by the mitochondrial electron transport chain, J Neurochem 80, 780–7 (2002).
Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R, Ceramide synthase mediates daunorubicin-induced apoptosis:Analternative mechanism for generating death signals, Cell 82, 405–14 (1995).
Colell A, Morales A, Fernandez-Checa JC, Garcia-Ruiz C, Ceramide generated by acidic sphingomyelinase contributes to tumor necrosis factor-alpha-mediated apoptosis in human colon HT-29 cells through glycosphingolipids formation. Possible role of ganglioside GD3, FEBS Lett 526, 135–41 (2002).
De Maria R, Rippo MR, Schuchman EH, Testi R, Acidic sphingomyelinase (ASM) is necessary for fas-induced GD3 ganglioside accumulation and efficient apoptosis of lymphoid cells, J Exp Med 187, 897–902 (1998).
Garcia-Ruiz C, Colell A, Morales A, Calvo M, Enrich C, Fernandez-Checa JC. Trafficking of ganglioside GD3 to mitochondria by tumor necosis factor—alpha, J Biol Chem 277, 36443–8 (2002).
Matyas GR, Morre DJ, Subcellular distribution and biosynthesis of rat liver gangliosides, Biochim Biophys Acta 921, 599-614 (1987)
Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T, Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses, Science 280, 1763–6 (1998).
Rusinol AE, Cui Z, Chen MH, Vance JE, A unique mitochondriaassociated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins, J Biol Chem 269, 27494–502 (1994).
Giammarioli AM, Garofalo T, Sorice M, Misasi R, Gambardella L, Gradini R, Fais S, Pavan A, Malorni W,GD3glycosphingolipid contributes to Fas-mediated apoptosis via association with ezrin cytoskeletal protein, FEBS Lett 506, 45–50 (2001).
Vyas KA, Patel HV, Vyas AA, Schnaar RL, Segregation of gangliosides GM1 and GD3 on cell membranes, isolated membrane rafts, and defined supported lipid monolayers, Biol Chem 382, 241–50 (2001).
Linardic CM, Hannun YA, Identification of a distinct pool of sphingomyelin involved in the sphingomyelin cycle, J Biol Chem 269, 23530–7 (1994).
Zhang P, Liu B, Jenkins GM, Hannun YA, Obeid LM, Expression of neutral sphingomyelinase identifies a distinct pool of sphingomyelin involved in apoptosis, J Biol Chem 272, 9609–12 (1997).
Kirschnek S, Paris F, Weller M, Grassme H, Ferlinz K, Riehle A, Fuks Z, Kolesnick R, Gulbins E, CD95-mediated apoptosis in vivo involves acid sphingomyelinase, J Biol Chem 275, 27316–23 (2000).
Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnick R, Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis, Cell 86, 189–99 (1996).
Kolesnick R, The therapeutic potential of modulating the ceramide/ sphingomyelin pathway, J Clin Invest 110, 3–8 (2002).
Liu P, Anderson RG, Compartmentalized production of ceramide at the cell surface, J Biol Chem 270, 27179–85 (1995).
Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E, CD95 signaling via ceramide-rich membrane rafts, J Biol Chem 276, 20589–96 (2001).
Wiegmann K, Schutze S, Machleidt T, Witte D, Kronke M, Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling, Cell 78, 1005–15 (1994).
Cifone MG, Roncaioli P, De Maria R, Camarda G, Santoni A, Ruberti G, Testi R, Multiple pathways originate at the Fas/APO-1 (CD95) receptor: Sequential involvement of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase in the propagation of the apoptotic signal, Embo J 14, 5859–68 (1995).
Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M, TNF activates NF-kappa B by phosphatidylcholinespecific phospholipase C-induced "acidic" sphingomyelin breakdown, Cell 71, 765–76 (1992).
Makin G, Dive C, Apoptosis and cancer chemotherapy, Trends Cell Biol 11, S22–6 (2001).
Bosch I, Croop J, P-glycoprotein multidrug resistance and cancer, Biochim Biophys Acta 1288, F37–54 (1996).
Sietsma H, Veldman RJ, Kok JW, The involvement of sphingolipids in multidrug resistance, J Membr Biol 181, 153–62 (2001).
Ogretmen B, Hannun YA, Updates on functions of ceramide in chemotherapy-induced cell death and in multidrug resistance, Drug Resist Updat 4, 368–77 (2001).
Senchenkov A, Litvak DA, Cabot MC, Targeting ceramide metabolism—A strategy for overcoming drug resistance, J Natl Cancer Inst 93, 347–57 (2001).
Marchell NL, Uchida Y, Brown BE, Elias PM, Holleran WM, Glucosylceramides stimulate mitogenesis in aged murine epidermis, J Invest Dermatol 110, 383–7 (1998).
Lavie Y, Cao H, Bursten SL, Giuliano AE, Cabot MC, Accumulation of glucosylceramides in multidrug-resistant cancer cells, J Biol Chem 271, 19530–6 (1996).
Liu YY, Han TY, Giuliano AE, Cabot MC, Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells, J Biol Chem 274, 1140–6 (1999).
Lavie Y, Cao H, Volner A, Lucci A, Han TY, Geffen V, Giuliano AE, Cabot MC, Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells, J Biol Chem 272, 1682–7 (1997).
Liu YY, Han TY, Giuliano AE, Hansen N, Cabot MC, Uncoupling ceramide glycosylation by transfection of glucosylceramide synthase antisense reverses adriamycin resistance, J Biol Chem 275, 7138–43 (2000).
Lucci A, Han TY, Liu YY, Giuliano AE, Cabot MC, Multidrug resistance modulators and doxorubicin synergize to elevate ceramide levels and elicit apoptosis in drug-resistant cancer cells, Cancer 86, 300–11 (1999).
Deng W, Li R, Guerrera M, Liu Y, Ladisch S, Transfection of glucosylceramide synthase antisense inhibits mouse melanoma formation, Glycobiology 12, 145–52 (2002).
Ferrari G, Anderson BL, Stephens RM, Kaplan DR, Greene LA, Prevention of apoptotic neuronal death by GM1 ganglioside. Involvement of Trk neurotrophin receptors, J Biol Chem 270, 3074–80 (1995).
Edsall LC, Pirianov GG, Spiegel S, Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation, J Neurosci 17, 6952–60 (1997).
Cavallini L, Venerando R, Miotto G, Alexandre A, Ganglioside GM1 protection from apoptosis of rat heart fibroblasts, Arch Biochem Biophys 370, 156–62 (1999).
Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS, Gray MO, Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts, Am J Physiol Heart Circ Physiol 282, H1970–7 (2002).
Ariga T, Jarvis WD, Yu RK, Role of sphingolipid-mediated cell death in neurodegenerative diseases, J Lipid Res 39, 1–16 (1998).
Hiraishi K, Suzuki K, Hakomori S, Adachi M, Le(y) antigen expression is correlated with apoptosis (programmed cell death), Glycobiology 3, 381–90 (1993).
Hakomori S, Bifunctional role of glycosphingolipids, Modulators for transmembrane signaling and mediators for cellular interactions, J Biol Chem 265, 18713–6 (1990).
Paller AS, Arnsmeier SL, Alvarez-Franco M, Bremer EG, Ganglioside GM3 inhibits the proliferation of cultured keratinocytes, J Invest Dermatol 100, 841–5 (1993).
Paller AS, Siegel JN, Spalding DE, Bremer EG, Absence of a stratum corneum antigen in disorders of epidermal cell proliferation: Detection with an anti-ganglioside GM3 antibody, J Invest Dermatol 92, 240–6 (1989).
Wang X, Rahman Z, Sun P, Meuillet E, George D, Bremer EG, Al-Qamari A, Paller AS, Ganglioside modulates ligand binding to the epidermal growth factor receptor, J Invest Dermatol 116, 69–76 (2001).
Ono M, Handa K, Withers DA, Hakomori S, Motility inhibition and apoptosis are induced by metastasis-suppressing gene product CD82 and its analogue CD9, with concurrent glycosylation, Cancer Res 59, 2335–9 (1999).
Kakugawa Y, Wada T, Yamaguchi K, Yamanami H, Ouchi K, Sato I, Miyagi T, Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression, Proc Natl Acad Sci USA 99, 10718–23 (2002).
Hakomori S, Aberrant glycosylation in cancer cell membranes as focused on glycolipids: Overview and perspectives, Cancer Res 45, 2405–14 (1985).
Hanai N, Nakamura K, Shitara K, Recombinant antibodies against ganglioside expressed on tumor cells, Cancer Chemother Pharmacol 46, S13–7 (2000).
Bitton RJ, Guthmann MD, Gabri MR, Carnero AJ, Alonso DF, Fainboim L, Gomez DE, Cancer vaccines: An update with special focus on ganglioside antigens, Oncol Rep 9, 267–76 (2002).
Pagnan G, Montaldo PG, Pastorino F, Raffaghello L, Kirchmeier M, Allen TM, Ponzoni M, GD2-mediated melanoma cell targeting and cytotoxicity of liposome-entrapped fenretinide, Int J Cancer 81, 268–74 (1999).
Li R, Villacreses N, Ladisch S, Human tumor gangliosides inhibit murine immune responses in vivo, Cancer Res 55, 211–4 (1995).
Bharti AC, Singh SM, Induction of apoptosis in bone marrow cells by gangliosides produced by a T cell lymphoma, Immunol Lett 72, 39–48 (2000).
Farina F, Cappello F, Todaro M, Bucchieri F, Peri G, Zummo G, Stassi G, Involvement of caspase-3 and GD3 ganglioside in ceramide-induced apoptosis in Farber disease, J Histochem Cytochem 48, 57–62 (2000).
Nakamura M, Tsunoda A, Furukawa Y, Sakai T, Saito M, Rapid internalization of exogenous ganglioside GM3 and its metabolism to ceramide in human myelogenous leukemia HL-60 cells compared with control ganglioside GM1, FEBS Lett 400, 350–4 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bektas, M., Spiegel, S. Glycosphingolipids and cell death. Glycoconj J 20, 39–47 (2003). https://doi.org/10.1023/B:GLYC.0000016741.88476.8b
Issue Date:
DOI: https://doi.org/10.1023/B:GLYC.0000016741.88476.8b