Abstract
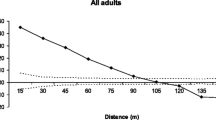
Three relatively isolated stands were used to study gene flow in European beech (Fagus sylvatica L.) in Northern Germany. Nine allozyme loci (Got-B, Idh-A, Lap-A, Mdh-B, Mdh-C, Mnr-A, 6-pgdh-A, Pgi-B and Pgm-A) were utilized for multilocus-genotyping adult trees and seeds. Expected heterozygosity (H e) ranged from 0.325 to 0.351 for the three stands. F ST revealed that there was small differentiation among stands (mean F ST= 0.013). The indirect estimates of gene flow (Nm) based on the mean F ST were high and the average Nm was 19.14. External gene flow by pollen ranged from 0.7 to 1.2% inferred from new alleles in seed samples. Moreover, paternity analysis was used to assess effective pollen dispersal by inferring paternity of offspring. The weighted mean distances of pollen dispersal for these three stands were 36.8 and 37.1 m based on simple exclusion procedure and most-likely method, respectively. Two of the trees in one stand had rare allozyme alleles (Lap-A1 and Idh-A4, respectively), which were used to directly measure pollen movement away from those trees. The frequency of the rare Lap and Idh alleles in seeds declines as the distance from the source tree increases. The weighted mean distance of pollen dispersal with rare allele Lap-A1 or Idh-A4 was 26.3 m.
Similar content being viewed by others
References
Adams, W. T., 1992. Gene dispersal within forest tree popula-tions. New Forests 6:217–240.
Adams, W. T. & D. S. Birkes, 1989. Mating patterns in seed orchards, pp. 75–86 in Proc. 20th South. For. Tree Improv. Conf., Charleston, South Carolina.
Adams, W. T. & D. S. Birkes, 1991. Estimating mating patterns in forest tree populations, pp. 157–172 in Biochemical Markers in the Population Genetics of Forest Trees, edited by H. H. Hattemer & S. Fineschi. S. P. B. Academic Publishing, The Hague.
Adams, W. T., A. R. Griffin & G. F. Moran, 1992. Using paternity analysis to measure pollen dispersal in plant populations. Am. Nat. 140:762–780.
Boshier, D. H., R. C. Michael & K. S. Bawa, 1995. Population genetics of Cordia alliodora (Boraginaceae), a neotropical tree. 3. Gene flow, neighborhood, and population substruc-ture. Am. J. Bot. 82:484–490.
Bradshaw, A. D., 1972. Some evolutionary consequences of being a plant. Evol. Biol. 5:25–47.
Burczyk, J., W. T. Admas & J. Y. Shimizu, 1996. Mating patterns and pollen dispersal in a natural knobcone pine (Pinus attenuata Lemmon). Heredity 77:251–260.
Campbell, D. R., 1991. Comparing pollen dispersal and gene flow in a natural population. Evolution 45:1965–1968.
Campbell, D. R., 1998. Multiple paternity in fruits of Ipomopsis aggregata (Polemoniaceae). Am. J. Bot. 85:1022–1027.
Comps, B., B. Thiebaut, L. Paule, D. Merzeau & J. Letouzey, 1990. Allozymic variability in beechwoods (Fagus sylvatica L. )over centrol Europeae:spatial differentiation among and within populations. Heredity 65:407–417.
Devlin, B. & N. C. Ellstrand, 1990. The development and application of a re ned method for estimating gene flow from angiosperm paternity analysis. Evolution 44:248–259.
Devlin, B., K. Roeder & N. C. Ellstrand, 1988. Fractional paternity assignment:theoretical development and comparison to other methods. Theor. Appl. Genet. 76:369–380.
Di-Giovanni, F. & P. G. Kevan, 1991. Factors affecting pollen dynamics and its importance to pollen contamination: a review. Can. J. Forest Res. 21:1155–170.
Dow, B. D. & M. V. Ashley, 1996. Microsatellite analysis of seed dispersal and parentage of saplings in bur oak, Quercus macrocarpa. Mol. Ecol. 5:615–627.
El-Kassaby, Y. A. & K. Ritland, 1986. Low levels of pollen contamination in a Douglas-r seed orchard as detected by allozyme markers. Silvae Genet. 35:224–229.
Ellstrand, N. C., 1984. Multiple paternity within fruits of the wild radish, Raphanus sativus. Am. Nat. 123:819–828.
Ellstrand, N. C. & D. L. Marshall, 1985. Interpopulation gene flow by pollen in wild radish, Raphanus sation, Am. Nat. 126:606–616.
Ennos, R. A., 1994. Estimating the relative rates of pollen and seed migration among plant populations. Heredity 72:250–259.
Govindaraju, D. R., 1988. Relationship between dispersal abil-ity and levels of gene flow in plants. Oikos 52:31–35.
Govindaraju, D. R., 1989. Estimates of gene flow in forest trees. Bio. J. Linn. Soc. 37:345–357.
Hamrick, J. L., 1991. Correlations between species traits and allozyme diversity: impflications for conserration biology, pp. 75–86 in Genetics and Conservation of Rare Plants, edited by D. A. Falk & K. E. Holsinger. Oxford University Press, New York.
Hamrick, J. L. & A. Schnabel, 1985. Understanding the genetic structure of plant populations. Some old problems and a new approach, pp. 50–70 in Population Genetics in Forestry. Lecture Notes in Biomathematics, Vol. 60, edited by H.-R. Gregorius. Springer-Verlag Berlin, Heidelberg, New York, Tokyo.
Hattemer, H. H., 1994. Die genetische Variation und ihre Bedeutung fűr Wald und Waldbaűme. Schweiz. Z. Forstwes 145:953–975.
Hattemer, H. H., 1995. Concepts and requirements in the conservation of forest genetic resources. Forest Genet 2: 125–134.
Hattemer, H. H., F. Bergmann & M. Ziehe, 1993. Einfu ¨hrung in die Genetik. J. D. Sauerländer 's Verlag, Frankfurt am Main, 2. Auflage.
Hattemer, H. H. & G. H. Melchior, 1993. Genetics and its application to tropical forestry, pp. 333–380 in Tropical Forestry Handbook. Vol. 1, edited by L. Pancel. Springer-Verlag, Berlin, Heidelberg.
Hattemer, H. H. & M. Ziehe, 1997. Genetic control of pheno-typic traits with relevance to gene conservation in trees–a survey of methods, pp. 135–148 in Perspectives of Forest Genetics and Tree Breeding in a Changing World, edited by C. Mátyás. IUFRO World Series Vol. 6, Sopron.
Konnert, M., M. Ziehe, U. Tröber, W. Maurer, A. Janßen, T. Sander, E. Hussendöfer & H. Hertel, 2000. Genetische Variation der Buche (Fagus sylvatica L.)in Deutschland: Gemeinsame Auswertung genetischer Inventuren u ¨ber verschiedene Bundesländer. [Genetic variation of beech (Fagus sylvatica L.)in Germany:joint evaluation of genetic inventories from several federal states.] Forst und Holz 55: 403–408.
Latta, R. G., Y. B. Linhart, D. Fleck & M. Elliot, 1998. Direct and indirect estimates of seed versus pollen movement within a population of ponderosa pine. Evolution 52: 61–67.
Ledig, F. T., 1986. Heterozygosity, heterosis, and tness in outbreeding plants, pp. 77–104 in Conservation Biology: The Science of Scarcity and Diversity, edited by M. E. Soule. Sinauer Sunderland, Mass.
Leonardi, S. & P. Menozzi, 1995. Genetic variability of Fagus sylvatica L. in Italy: the role of postglacial recolonization. Heredity 75:35–44.
Levin, D. A., 1981. Dispersal versus gene flow in plants. Ann. Missouri Bot. Gard. 68:233–253.
Marshall, T. C., J. Slate, L. E. B. Kruuk & J. M. Pemberton, 1998. Statistical con dence for likelihood-based paternity inference in natural populations. Mol. Ecol. 7:639–655.
Meagher, T. R., 1986. Analysis of paternity within a natural population of Chamaelirium luteum. 1. Identification of most-likely male parents. Am. Nat. 128:199–215.
Meagher, T. R. & E. Thomson, 1987. Analysis of parentage for naturally established seedlings of Chamaelirium luteum (Liliaceae). Ecology 68:803–812.
Merzeau, D., B. Comps, B. Thiebaut & J. Letouzey, 1994. Estimation of Fagus sylvatica L mating system parameters in natural populations. Ann. Sci. Forest 5 1: 163–173.
Műller, G., 1977. Cross-fertilization in a conifer stand inferred from enzyme gene markers in seeds. Silvae Genet. 26:223–226.
Műller-Starck, G., P. Baradat & F. Bergmann, 1992. Genetic variation within European tree species. New Forests 6: 23–47.
Műller-Starck, G. & R. Starke, 1993. Inheritance of isoenzymes in European beech (Fagus sylvatica L.). J. Heredity 84: 291–296.
Műller-Starck, G. & M. Ziehe, 1991. Genetic variation in populations of Fagus sylvatica L., Quercus robur L. and Q. petraea Liebl. (in Germany), pp. 125–149 in Genetic Variation in European Populations of Forest Trees, edited by G. Műller-Starck & M. Ziehe. Frankfurt am Main. J. D. Sauerländer 's Verlag.
Műller-Starck, R., 1996. Genetische Aspekte der Reproduktion der Buche (Fagus sylvatica L. )unter Berűcksichtigung waldbaulicher Gegebenheiten. Berichte des Forschungszen-trums Waldo ¨kosysteme, Reihe A, Bd. 135. Göttingen, pp. 103.
Namkoong, G. & H.-R. Gregorius, 1985. Conditions for protected polymorphisms in subdivided plant populations. 2. Seed versus pollen migration. Am. Nat. 125:521–534.
Paule, L., D. Gömöry & J. Vyšnŷ, 1995. Genetic diversity and differentiation of beech populations in eastern Europe, pp. 159–167 in Genetics and Silviculture of Beech. Proceedings from the 5th Beech Symposium of IUFRO. Forskiningsse-rien Nr. 11, edited by S. F. Madsen. Danish Forest and Landscape Research Institute, Hørsholm.
Schnabel, A., 1988. Population genetic structure and gene flow in Gleditsia triacanthos. Ph. D. Dissertation. University of Kansas, Lawrence.
Schnabel, A. & J. L. Hamrick, 1995. Understanding the population genetic structure of Gleditsia triacanthos L.: the scale and pattern of pollen gene flow. Evolution 49: 921–931.
Schuster, W. F. & J. B. Mitton, 2000. Paternity and gene dispersal in limber pine (Pinus flexilis fames). Heredity 84: 348–361.
Slate, J., T. Marshall & J. Pemberton, 2000. A retrospective assessment of the accuracy of the paternity inference program CERVUS. Mol. Ecol. 9:801–808.
Slatkin, M., 1987. Gene flow and the geographic structure of natural populations. Science 236:787–792.
Smouse, P. E., R. J. Dyer, R. D. Westfall & V. L. Sork, 2001. Two-generation analysis of pollen flow across a landscape. I. Male gamete heterogeneity among females. Evolution 55: 260–271.
Stacy, E. A., J. L. Hamrick, J. D. Nason, S. P. Hubbell, R. B. Foster & R. Condit, 1996. Pollen dispersal in low-density populations of three neotropical tree species. Am. Nat. 148: 275–298.
Streif, R., T. Labbe, R. Bacilieri, H. Steinkellner, J. Glossl & A. Kremer, 1998. Within population genetic structure in Quercus robur L. and Quercus petraea (Matt.). Mol. Ecol. 7: 317–328.
Troggio, M., E. Dimasso, S. Leonardi, M. Ceroni, G. Bucci, P. Piovani & P. Menozzi, 1996. Inheritance of RAPD and I-SSR markers and population parameters estimation in European beech (Fagus sylvatica L.). Forest Genet. 3: 173–181.
Turner, M. E., J. C. Stephens & W. W. Anderson, 1982. Homozygosity and patch structure in plant populations as a result of nearest-neighbor pollination. Proc. Nat. Acad. Sci. USA 79:203–207.
Wang, K. S. & H. H. Hattemer, 2001. Dispersal of seed and effective pollen in small stands of European beech (Fagus sylvatica L.), pp. 259–269 in Genetic Response of Forest Systems to Changing Environmental Conditions, edited by G. Műller-Starck & R. Schubert. Kluwer Academic Publishers, Dordrecht.
Voelker, R. A., H. E. Schaffer & T. Mukai, 1980. Spontaneous allozyme mutations in Drosophila Melanogaster: rate of occurrence and nature of the mutants. Genetics 94: 961–968.
Waser, N. M., 1993. Population structure, optimal outbreeding and assortative mating in angiosperms, pp. 139–199 in The Natural History of Inbreeding and Outbreeding:Theoret-ical and Empirical Perspectives, edited by N. Wilmsen-Thornhill. University of Chicago Press, Chicago.
Wheeler, N. C., W. T. Adams & J. L. Hamrick, 1992. Pollen distribution in wind-pollinated seed orchards, in Pollen Management Handbook, Vol. 2, edited by D. L. Bramlett, G. R. Askew, T. D. Blush, F. E. Bridgwater & J. B. Jett. USDA Forest Service Agric. Bulletin.
Weir, B. S., 1996. Genetic Data Analysis II. Sinauer Associa-tion, Sunderland, MA, USA.
Wright, S., 1931. Evolution in Mendelian populations. Genetics 16:97–159.
Wright, S., 1938. Size of population and breeding structure in relation to evolution. Science 87:430–431.
Wright, S., 1951. The genetic structure of populations. Ann. Eugen. 15:323–354.
Yeh, F. C., R. C. Yang & T. Boyle, 2000. Population Genetic Analysis POPGENE Version 1. 32: A Quick User 's Guide. A joint Project Development by University Alberta, Can-ada and Center for International Forestry Research, USA.
Ziehe, M., 1998. Anpassungsprozesse auf Populationsebene, in Abschlussbericht 1998 des Forschungszentrums Waldo ¨ko-systeme. Teil 1:115–141. ISSN 0939-1339.
Ziehe, M. & H. H. Hattemer, 1998. The signi cance of heterozygosity in tree breeding and gene conservation. Forest Tree Improvement 26:25.
Ziehe, M., H.-G. Gregorius & G. Műller-Starck, 1990. Zur Bedeutung der Heterozygotie fűr die dynamische Genkon-servierung, pp. 46–57 in Erhaltung forstlicher Genressour-cen, edited by H. H. Hattemer. Schriften aus der Forstl. Fakultät d. Univ. Göttingen, Bd. 98.
Ziehe, M., R. Starke, H. H. Hattemer & J. Turok, 1998. Genotypische Strukturen in Buchen-Altbeständen und ihren Samen. Allgem. Forst-u. Jagdztg. 69:91–99.
Ziehe, M., H. H. Hattemer, R. Műller-Starck & G. Műller-Starck, 1999. Genetic structures as indicators for adapta-tion and adaptational potentials, pp. 75–89 in Forest Genetics and Sustainability, edited by C. Mátyás. Kluwer Academic Publishers, Dordrecht, The Netherlands.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, K. Gene Flow in European Beech (Fagus sylvatica L.). Genetica 122, 105–113 (2004). https://doi.org/10.1023/B:GENE.0000040999.07339.d4
Issue Date:
DOI: https://doi.org/10.1023/B:GENE.0000040999.07339.d4