Abstract
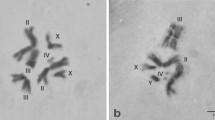
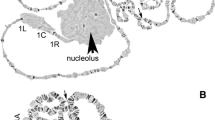
In situ hybridization was used to determine the physical location of the Hsp70 genes in salivary polytene chromosomes of Anopheles darlingi from Manaus and Macapá, Brazil, and to assess the usefulness of the Hsp70 locus as a genetic marker in A. darlingi populations. In both populations, the double markings corresponding to the Hsp70-12A and Hsp70-14A genes were located on the right arm of chromosome 2. The Hsp70 locus was considered to be an excellent marker for studying chromosomal evolution and relationships among A. darlingi populations.
Similar content being viewed by others
References
Atherton, D. & J.G. Gall, 1972. Salivary gland squashes for in situ nucleic acid hybridization studies. Drosophila Inf. Serv. 49: 131–133.
Benedict, M.Q., A.F. Cockburn & J.A. Seawright, 1993. The Hsp70 heat-shock gene family of the mosquito Anopheles albimanus. Insect Mol. Biol. 2: 93–102.
Bonorino, C.B.C., M. Pereira, C.E.V. Alonso, V.L.S. Valente & E. Abdelhay, 1993. In situ mapping of the hsp70 locus in seven species of the willistoni group of Drosophila. Rev. Brasil. Genet. 16: 561–571.
Conn, J.E., M.G. Freitas-Sibajev, S.L.B. Luz & H. Momen, 1999. Molecular population genetics of the primary neotropical malaria vector Anopheles darlingi using mtDNA. J. Am. Mosq. Cont. Assoc. 15: 468–474.
De Arruda M., M.B. Carvalho, R.S. Nussenzweig, M. Mararic, A.W. Ferreira & A.H. Cochrane, 1986. Potential vectors of malaria and their different susceptibility to Plasmodium falciparum and Plasmodium vivax in northern Brazil identified by immunoassay. Am. J. Trop. Med. Hyg. 35: 873–881.
Drosopoulou, E., I. Konstantopoulou & Z.G. Scouras, 1996. The heat shock genes in the Drosophila montium subgroup: chromosomal localization and evolutionary implications. Chromosoma 105: 104–110.
Faran, M.E. & K.J. Linthicum, 1981. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae). Mosq. Syst. 13: 1–81.
Feder, M.E. & R.A. Krebs, 1998. Natural and genetic engineering of thermotolerance in Drosophila melanogaster. Am. Zool. 38: 503–517.
Foote, R.H. & D.R. Cook, 1959. Mosquitoes of Medical Importance. Agricultural Handbook. Vol. 152, U.S. Department of Agriculture, Washington, DC, pp. 1–158.
Forattini, O.P., 1962. Entomologia Médica. Vol. 1, Faculdade de Higiene e Saú de Pú blica da USP, Sã o Paulo, 662 p.
Freitas-Sibajev, M.G.R, J. Conn, S.E. Mitchell, A.F. Cockburn, J.A. Seawright & H. Momen, 1995. Mitochondrial DNA and morphological analyses of Anopheles darlingi populations from Brazil (Diptera: Culicidae). Mosq. Syst. 27: 78–99.
French, W.L., R.H. Baker & J.B. Kitzmiller, 1962. Preparation of mosquito chromosomes. Mosq. News 22: 377–383.
Gabai, V.L. & M.Y. Sherman, 2002. Interplay between molecular chaperones and signaling pathways in survival of heat shock. J. Appl. Physiol. 92: 1743–1748.
Gall, J.G. & M.L. Pardue, 1969. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc. Natl. Acad. Sci. USA 63: 378–383.
Graziosi, C., R.K. Sakai, P. Romans, L.H. Miller & T.E. Wellems, 1990. Method for in situ hybridization to polytene chromosomes from ovarian nurse cells of Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 27: 905–912.
Guedes, A.S., E.M. Amorim & G. Schreiber, 1957. Análise dos cromossomos salivares em anofelinos brasileiros. Rev. Bras. Malar. D. Trop. 9: 247–250.
Holt, R.A., G.M. Subramanian, A. Halpern, G.G. Sutton, R. Charlab, D.R. Nusskern, P. Wincker, A.G. Clark, J.M. Ribeiro, R. Wides, S.L. Salzberg, B. Loftus, M. Yandell, W.H. Majoros, D.B. Rusch, Z. Lai, C.L. Kraft, J.F. Abril, V. Anthouard, P. Arensburger, P.W. Atkinson, H. Baden, V. de Berardinis, D. Baldwin, V. Benes, J. Biedler, C. Blass, Bolanos, D. Boscus, M. Barnstead, S. Cai, A. Center, K. Chatuverdi, G.K. Christophides, M.A. Chrystal, M. Clamp, A. Cravchik, V. Curwen, A. Dana, A. Delcher, I. Dew, C.A. Evans, M. Flanigan, A. Grundschober-Freimoser, L. Friedli, Z. Gu, P. Guan, R. Guigo, M.E. Hillenmeyer, S.L. Hladun, J.R. Hogan, Y.S. Hong, J. Hoover, O. Jaillon, Z. Ke, C. Kodira, E. Kokoza, A. Koutsos, I. Letunic, A. Levitsky, Y. Liang, J.J. Lin, N.F. Lobo, J.R. Lopez, J.A. Malek, T.C. McIntosh, S. Meister, J. Miller, C. Mobarry, E. Mongin, S.D. Murphy, D.A. O'Brochta, C. Pfannkoch, R. Qi, M.A. Regier, K. Remington, H. Shao, M.V. Sharakhova, C.D. Sitter, J. Shetty, T.J. Smith, R. Strong, J. Sun, D. Thomasova, L.Q. Ton, P. Topalis, Z. Tu, M.F. Unger, B. Walenz, A. Wang, J. Wang, M. Wang, X. Wang, K.J. Woodford, J.R. Wortman, M. Wu, A. Yao, E.M. Zdobnov, H. Zhang, Q. Zhao, S. Zhao, S.C. Zhu, I. Zhimulev, M. Coluzzi, A. della Torre, C.W. Roth, C. Louis, F. Kalush, R.J. Mural, E.W. Myers, M.D. Adams, H.O. Smith, S. Broder, M.J. Gardner, C.M. Fraser, E. Birney, P. Bork, P.T. Brey, J.C. Venter, J. Weissenbach, F.C. Kafatos, F.H. Collins & S.L. Hoffman, 2002. The genome sequence of the malaria mosquito Anopheles gambiae. Science 298: 129–149.
Ish-Horowicz, D. & S.M. Pinchin, 1980. Genomic organization of the 87A7 and 87C1 heat-induced loci of Drosophila melanogaster. J. Mol. Biol. 142: 231–245.
Konstantopoulou, I., N. Nikolaidis & Z.G. Scouras, 1998. The hsp70 locus of Drosophila auraria (montium subgroup) is single and contains copies in a conserved arrangement. Chromosoma 107: 577–586.
Kreutzer, R.D., J.B. Kitzmiller & E. Ferreira, 1972. Inversion polymorphism in the salivary gland chromosomes of Anopheles darlingi Root. Mosq. News 32: 555–565.
Kreutzer, R.D., J.B. Kitzmiller & M.G. Rabbani, 1975. The salivary gland chromosomes of Anopheles argyritarsis compared with those of certain other species in the subgenus Nyssorhynchus. Mosq. News 35: 354–365.
Kumar, V. & F.H. Collins, 1994. A technique for nucleic acid in situ hybridization to polytene chromosomes of mosquitoes in the Anopheles gambiae complex. Insect Mol. Biol. 3: 41–47.
Leigh-Brown, A.J. & D. Ish-Horowicz, 1981. Evolution of the 87A and 87C heat-shock loci in Drosophila. Nature 290: 677–682.
Linthicum, K.J., 1988. A revision of the Argyritarsis section of the subgenus Nyssorhynchus of Anopheles (Diptera: Culicidae). Mosq. Syst. 20: 98–271.
Manguin, S., R.C. Wilkerson, J.E. Conn, Y. Rubio-Palis, J.A. Danoff-Burg & D.R. Roberts, 1999. Population structure of the primary malaria vector in South America, Anopheles darlingi, using isozyme, random amplified polymorphic DNA, internal transcribed spacer 2, and morphologic markers. Am. J. Trop. Med. Hyg. 60: 364–376.
Molto, M.D., L. Pascual, M.J. Martinez-Sebastian & R. de Frutos, 1992. Genetic analysis of heat shock response in three Drosophila species of the obscura group. Genome 35: 870–880.
Narang, S.K. & J.A. Seawright, 1993. In situ hybridization mapping of histone genes in Anopheles albimanus. J. Am.Mosq. Control Assoc. 9: 147–149.
Peters, F.P.A.M.N., N.H. Lubsen & P.J.A. Sondermeijer, 1980. Rapid sequence divergence in a heat shock locus of Drosophila. Chromosoma 81: 271–280.
Rafael, M.S. & W.P. Tadei, 2003. Location of ribosomal genes in the chromosomes of Anopheles darlingi and Anopheles nuneztovari (Diptera, Culicidae) from the Brazilian Amazon. Mem. Inst. Oswaldo Cruz 98(5): 629–635.
Rosa-Freitas, M.G., G. Broomfield, A. Priestmann, P. Milligan, H. Momen & D.H. Molyneux, 1992. Studies on cuticular components, isoenzymes and behaviour of 3 populations of Anopheles darlingi from Brazil. J. Am. Mosq. Control Assoc. 8: 357–366.
Santos, J.M.M., J.A. Lobo, W.P. Tadei & E.P.B. Contel, 1999. Intrapopulational genetic differentiation in Anopheles (N.) darlingi Root, 1926 (Diptera: Culicidae) in the Amazon region. Genet. Mol. Biol. 22: 325–331.
Schreiber, G. & A.S. Guedes, 1959. Estudo comparativo do cromosoma X em algumas espécies de Anopheles do sub-gen. Nyssorhynchus (Dipt. Culic.). Ciê nc. Cult. 11: 128–129.
Schreiber, G. & A.S. Guedes, 1960. Perspectivas citoló gicas na sistemática dos anofelinos (S.G. Nyssorhynchus). Rev. Bras. Mal. D. Trop. 12: 355–358.
Tadei, W.P., J.M.M. Santos & M.G. Rabbani, 1982. Biologia de anofelinos amazô nicos. V. Polimorfismo cromossô mico de Anopheles darlingi Root (Diptera, Culicidae). Acta Amaz. 12: 353–369.
Tadei, W.P., J.M.M. Santos & A.S. Cunha, 1984. Sobre o polimor-fismo cromossô mico de Anopheles darlingi Root (Diptera, Culicidae). Ciê nc Cult. 36: 845.
Tadei, W.P., B. Dutary-Thatcher, J.M.M. Santos, V.M. Scarpassa, I.B. Rodrigues & M.S. Rafael, 1998. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 59: 325–335.
Toaldo, C.B., M. Steindel, M.A. Sousa & C.C. Tavares, 2001. Molecular karyotype and Chromosomal localization of genes encoding ?-tubulin, cysteine proteinase, hsp70 and actin in Trypanosoma rangeli. Mem. Inst. Oswaldo Cruz 96: 113–121.
Zacharopoulou, A.M. Frisardi, C. Savakis, A.S. Robinson, P. Tolias, M. Konsolaki, K. Komitopoulou & F.C. Kafatos, 1992. The genome of the Mediterranean fruit fly Ceratitis capitata: localization of molecular markers by in situ hybridization to salivary gland polytene chromosomes.Chromosoma 101: 448–455.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rafael, M.S., Tadei, W.P. & Hunter, F.F. The Physical Gene Hsp70 Map on Polytene Chromosomes of Anopheles darlingi from the Brazilian Amazon. Genetica 121, 89–94 (2004). https://doi.org/10.1023/B:GENE.0000019959.45267.d7
Issue Date:
DOI: https://doi.org/10.1023/B:GENE.0000019959.45267.d7