Abstract
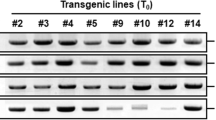
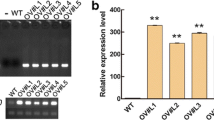
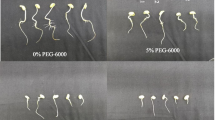
Transgenic soybean, containing a L-Δ1-Pyrroline-5-carboxylate reductase (P5CR) gene in sense or antisense orientation, was compared with untransformed control soybean while subjected to drought stress, through evaluation of physiological techniques. The significant higher relative water content (RWC) of the sense plants, especially after 8 days stress, coincides with much higher free proline levels compared to control and antisense plants. It was implied that some of this free proline might be a result of protein degradation and therefore proline dehydrogenase (PDH) enzyme analysis was performed. PDH activity was highest in the antisense plants, followed by control plants and least the sense plants. This confirmed that some free proline measured in antisense plants were degradation products. In the sense plants, the initial changes after the drought stress were largely due to a rise in reducing sugars. In the antisense plants the first reaction was an increase in sucrose. The fructose and glucose levels were highest in the sense plants at 4 and 6 dww. Molecular analysis of transgenic plants confirms the presence of between one and five copies of the P5CR gene in the test plants. The sense plants behaved more tolerant to the drought stress and experienced the highest RWC, highest free proline levels, higher or similar P5CR but lower PDH enzyme activity compared to antisense and control plants. These results justify the hypothesis that the sense transgenic plants reacted more drought tolerant than the control and antisense plants.
Similar content being viewed by others
References
Bates, L.S., R.P. Waldren & I.D. Teare, 1973. Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207.
Bianco, R.L., M. Rieger & S.-J.S. Sung, 2000. Effect of drought on sorbitol and sucrose metabolism in sinks and sources of peach. Physiologia Plantarum 108: 71–78.
Binzel, M.L., P.M. Hasegawa, D. Rhodes, S. Handa, A.K. Handa & R.A. Bressan, 1987. Solute accumulation in tobacco cells adapted to NaCl. Plant Physiol 84: 1408–1415.
Bohnert, H.J. & B. Shen. Transformation and compatible solutes. Scientia Horticulturae 78: 237–260.
Csonka, L.N., 1981. Proline overproduction results in enhanced os-motolerance in Salmonella thyphimurium. Mol Gen Genet 182: 82–86.
De Ronde, J.A., W.A. Cress & A. Van der Mescht, 2001. Agrobac-terium mediated transformation of soybean seed with the GUS-INT marker gene. South African J Sci 97: 421–424.
De Ronde, J.A., W.A. Cress, G.H.J. Krüger, R.J. Strasser & J. Van Staden, 2004a. Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene during heat and drought stress. J Plant Physiol, in press.
De Ronde, J.A., W.A. Cress & J. Van Staden, 2004b. Phenotypic evaluation for drought tolerance of transgenic soybean. South African J Plant Soil, submitted.
De Roover, J., K. Vandenbranden, Van Laere & W. Van den Ende, 2000. Drought induces fructan synthesis and 1-SST in roots and leaves of chicory seedlings Cichorium intybus L. Planta 210: 808–814.
Dellaporta, S.L., J. Wood & J.B. Hicks, 1983. A plant DNA minipreparation: Version II. Plant Mol Biol Reporter 14: 19–21.
Devlin, R.M., 1975. Plant Physiology, 3rd edn, Carbohydrates, pp. 129–152. D Van Nostrand Company.
Edwards, K.C., C. Johnstone & C. Thompson, 1991. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19: 1349.
Hall, A.E., 2001. Crop Responses to Environment. CRC Press, 232 pp.
Hare, P.D., W.A. Cress & J. Van Staden, 1998. Dissecting roles of osmolyte accumulation during stress. Plant Cell Environ 21: 535–554.
Lawlor, D.W., 2002. Limitation to photosynthesis in water stressed leaves: Stomata vs. metabolism and the role of ATP. Annals Bot 89: 871–885.
Kerepesi, I., G. Galiba & E. Banyai, 1998. Osmotic and salt stresses induced differential alteration in water soluble carbohydrate con-tent in wheat seedlings. J Agric Food Chem 46: 5347–5354.
Koster, K.L. & A.C. Leopold, 1988. Sugars and desiccated tolerance in seed. Plant Physiol 96: 302–304.
Mattioni, C., N.G. Lacerenza, A. Troccoli, A.M. De Leonardis & N. Di Fonzo, 1997. Water and salt stress-induced alterations in pro-line metabolism of Triticum durum seedlings. Physiol Plantarum 101: 787–792.
McKersie, B.D. & Y.Y. Leshan, 1994. Stress and Stress Coping in Cultivated Plants. Kluwer Academic Publishers, London.
Muller, J., T. Boller & A. Wiemken, 1996. Pools of non structural car-bohydrates in soybean root nodules during water stress. Physiol Plantarum 98: 723–730.
Nilsen, E.T., D.M. Orcutt, 1996. Water limitations. In: Physiology of Plants under Stress. John Wiley and Sons Inc., pp. 322–361.
Peng, Z., Lu, Q. & D.P. Verma, 1996. Reciprocal regulation of delta 1-pyrroline-5-carboxylate synthetase & proline dehydrogenase genes controls proline levels during and after osmotic stress in plants. Mol Gen Genet 253: 334–341.
Rayapati, P.J. & C.R. Stewart, 1991. Solubilization of proline dehy-drogenase from maize Zea mays L. Plant Physiol 95: 787–791.
Sairam, R.K., K. Veerabhadra Rao & G.C. Strivastava, 2002. Dif-ferential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163: 1037–1046.
Toroser, D. & S.C. Huber, 1997. Protein phosphorylation as a mecha-nism for osmotic stress activation of sucrose phosphate synthetase in spinach leaves. Plant Physiol 114: 947–955.
Vagujfalvi, A., I. Kerepesi, G. Galiba, T. Tischner & J. Sutka, 1999. Frost hardiness depending on carbohydrate changes during cold acclimation in wheat. Plant Sci 144: 85–92.
Yeh, G.C. & J.M. Phang, 1988. Stimulation of phosphoribosyl py-rophosphate and purine nucleotide production by pyrroline-5-carboxylate in human erythrocutes. J Biol Chem 263: 13083–13089.
Zhu, B., J. Su, M. Chang, D.P.S. Verma, Y.-L., Fan & R. Wu, 1998. Overexpression of a 1-pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water-and salt-stress in transgenic rice. Plant Sci 139: 41–48.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Ronde, J., Laurie, R., Caetano, T. et al. Comparative study between transgenic and non-transgenic soybean lines proved transgenic lines to be more drought tolerant. Euphytica 138, 123–132 (2004). https://doi.org/10.1023/B:EUPH.0000046806.68554.5b
Issue Date:
DOI: https://doi.org/10.1023/B:EUPH.0000046806.68554.5b