Abstract
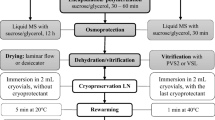
A cryopreservation process usingencapsulation/dehydration technique was setup for apical meristem-tips of invitro plantlets of `paradise tree' (Melia azedarach L. var. gigantea,clone `El dorado'). Apical meristem-tipswere cultured for one day on MS basalmedium with 2 μM BA and 0.5 μM IBAand encapsulated with 3% sodium alginate.The highest shoot proliferation rate aftercryopreservation was obtained whenencapsulated apical meristem-tips werepregrown for 3 days in liquid medium with0.5, 0.75 and 1 M of sucrose for 24 hoursprogressively, desiccated for 5 hours withsilicagel followed by rapid or slowcooling. Survival after freezing in liquidnitrogen ranged between 67–83% andshoot proliferation ranged between 43–60%. This cryopreservation treatmentpreserved genetic stability, when it wasevaluated using the electrophoreticpatterns of nine isozyme systems and RAPDprofiles.
Similar content being viewed by others
References
Aronen, T.S., J. Krajnakova, H.M. Hägman & L.A. Ryynänen, 1999. Genetic fidelity of cryopreserved embryogenic cultures of open-pollinated Abies cephalonica. Plant Science 142: 163–172.
Arulsekar, S. & D. Parfitt, 1986. Isozyme analysis procedures for stone fruits, almond, grape, walnut, pistachio and fig.HortScience 21: 928–933.
Ashmore, S.E., 1997. Current in vitro conservation techniques. In: F. Engelmann (Ed.), Status Report on the Development and Application of in vitro Techniques for the Conservation and Use of Plant Genetic Resources, pp. 5–18. IPGRI. Rome, Italy.
Bernard, F., H. Shaker-Bazarnov & B. Kaviani, 2002. Effects of salicylic acid on cold preservation and cryopreservation of encapsulated embryonic axes of Persian lilac (Melia azedarach L.). Euphytica 123: 85–88.
Blakesley, D., T. Percival, M.H. Bhatti & G.G. Henshaw, 1997. A simplified protocol for cryopreservation of embryogenic tissue of sweet potato (Ipomoea batatas (L.) Lam) utilizing sucrose preculture only. CryoLetters 18: 77–80.
De Loose, M. & G. Gheysen, 1995. Identification methods based on molecular techniques. Technical Bulletin of International Union for the Protection of new varieties of plants 3/2: 1–22.
Domecq, C.M., 1988. Cultivo in vitro de yemas axilares de paraíso gigante (Melia azedarach L. var. gigantea). Phyton 48: 33–42.
Dumet, D., F. Engelmann, N. Chabrillange, Y. Duval & J. Dereuddre, 1993. Importance sucrose for the acquisition of tolerance to desiccation and cryopreservation of oil palm somatic embryos. CryoLetters 14: 243–250.
Edwards, A., C. Johnstone & C. Thompson, 1991. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucl Acids Res 19: 1349.
Engelmann, F., 1997. In vitro conservation methods. In: J.A. Callow, B.V. Ford-Lloyd & H.J. Newbury (Eds.), Biotechnology and Plant Genetic Resources, pp. 119-161.
Fabre, J. & J. Dereuddre, 1990. Encapsulation-dehydration: a new approach to cryopreservation of Solanum shoot-tips. CryoLetters 11: 413–426.
González-Arnao, M.T., T. Moreira & C. Urra, 1996. Importance of pregrowth with sucrose and vitrification for the cryopreservation of sugarcane apices using encapsulation-dehydration. CryoLetters 17: 141–148.
González-Arnao, M.T., F. Engelmann, C. Urra, M. Morenza & A. Rios, 1998. Cryopreservation of Citrus apices using the encapsulation-dehydration technique. CryoLetters 19: 177–182.
Hägman, H.M., L.A. Ryynänen, T.S. Aronen & J. Krajnakova, 1998. Cryopreservation of embryogenic cultures of Scots pine. Plant Cell, Tissue and Organ Culture 54: 45–53.
Hirai, D. & A. Sakai, 2000. Cryopreservation of in vitro-grown meristems of potato (Solanum tuberosum L.) by encapsulationvitrification. In: F. Engelmann & H. Takagi (Eds.), Cryopreservation of Tropical Plant Germplasm. Current Research Progress and Application, pp. 205–211. JIRCAS-IPGRI, Rome, Italy.
Kartha, K.K., L. Leung & K. Pahl, 1980. Cryopreservation of strawberry meristems and mass propagation of plantlets. J Amer Soc Hortic Sci 105: 481–484.
Kunkel, G., 1978. Melia azedarach Linné-Persian lilac. In: W. Junk (Ed.), Flowering Trees in Subtropical Gardens, pp. 260–261. Dr. W. Junk bv publisher, The Hague.
Laemmli, U.K, 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Leonardis, R.F.J., H.R. Mangieri, J.C. Tinto, A. Alonzo & H.R. Reuter, 2000. In: Celulosa Argentina S.A. (Ed.), El nuevo libro del árbol. Especies exóticas de uso ornamental. Tomo 3, pp. 88–89. El Ateneo, Buenos Aires, Argentina.
Malaurie, B., M.F. Trouslot, F. Engelmann & N. Chabrillange, 1998. Effect of pretreatment conditions on the cryopreservation of in vitro-cultured yam (Dioscorea alata 'Brazo fuerte' and D. bulbifera 'Nouméa imboro') shoot apices by encapsulationdehydration. CryoLetters 19: 15–26.
Mangieri, H.R. & J.C. Tinto, 1977. In: Celulosa Argentina S.A. (Ed.), Libro del árbol. Esencias forestales no autóctonas cultivadas en la Argentina de aplicación ornamental y/o industrial, Tomo 3, pp. 61–62. Impresiones Ramos Mejía S.A., Buenos Aires, Argentina.
Marin, M.L., Y. Gogorcena, J. Ortiz & N. Duran-Vila, 1993. Recovery of whole plants of sweet orange from somatic embryos subjected to freezing thawing treatments. Plant Cell, Tissue and Organ Culture 34: 27–33.
Marzalina, M. & Z.N.A. Nashatul, 2000. Cryopreservation of some Malaysian tropical urban forestry species. In: F. Engelmann & H. Takagi(Eds.), Cryopreservation of Tropical Plant Germplasm, pp. 465–467. IPGRI, Rome, Italy.
Mroginski, L.A., W.M. Roca & K.K. Kartha, 1991. Crioconservación de germoplasma. In: W.M. Roca & L.A. Mroginski (Eds.), Cultivo de tejidos en la agricultura: fundamentos y aplicaciones, pp. 715–730. CIAT, Cali, Colombia.
Murashige, T. & F. Skoog, 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum 15: 473–497.
Nardo, E.A.B, A.S. Costa & A.L. Lourencao, 1997. Melia azedarach extract as an antifeedant to Bemisia tabaci (Homoptera: Aleyrodidae). Florida Entomologist 80: 92–94.
Paulet, F., F. Engelmann & J.Ch. Glaszmann, 1993. Cryopreservation of apices of in vitro plantlets of sugarcane (Saccharum sp. hybrids) using encapsulation-dehydration. Plant Cell Rep 12: 525–529.
Rohlf, F.J., 1994. NTSYS-pc Numerical Taxonomy and Multivariate Analysis System. Exeter software, New York.
Soltis D., C. Haufler, D. Darrow & U. Gastony, 1983. Starch gel electrophoresis of ferns: a compilation of grinding buffers, gel and electrode buffers, and staining schedules. Amer Fern J 73: 9–27.
Stuber, C.W., J.F. Wendel, M.M. Goodman & J.S.C. Smith, 1988. Techniques and scoring procedures for starch gel electrophoresis and enzymes from maize (Zea mays L.). Techn Bull North Carolina Agric Res Serv 286: 1–87.
Towill, L.E., 1984. Survival of ultra-low temperatures of shoot-tips from Solanum tuberosum groups andigena, phureja, stenotomum and other tuber-bearing Solanum species. CryoLetters 5: 319–326.
Vila, S., A. Scocchi & L. Mroginski, 2002. Plant regeneration from shoot apical meristems of Melia azedarach L. (Meliaceae). Acta Physiologiae Plantarum 24: 195–199.
Williams, J.G.K., A.R. Kubelik, K.J. Livak, J.A. Rafalski & S.V. Tingey, 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl Acids Res 18: 6531–6535.
Rights and permissions
About this article
Cite this article
Scocchi, A., Faloci, M., Medina, R. et al. Plant recovery of cryopreserved apical meristem-tips of Melia azedarach L. using encapsulation/dehydration and assessment of their genetic stability. Euphytica 135, 29–38 (2004). https://doi.org/10.1023/B:EUPH.0000009538.01279.d3
Issue Date:
DOI: https://doi.org/10.1023/B:EUPH.0000009538.01279.d3