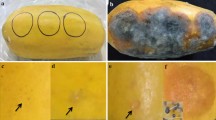
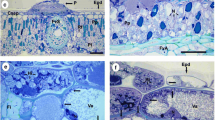
Abstract
Ultrastructural aspects of host–parasite interactions were investigated in fruits and leaves of citrus (satsuma mandarin) infected with Elsinoe fawcettii. Fungal infection induced host tissues to form cork layers bordering the necrotic areas below the infected sites. The cork layers were composed of compact host cells with convoluted cell walls and alternating lamellations, indicating ligno–suberized tissues in the wound periderm. No host tissues below the cork layers were invaded by hyphae. Hyphae grew intercellularly and intracellularly, often causing hypertrophy and compartmentalization of infected host cells. Also, host cells adjacent to invading hyphae showed accumulation of electron-dense materials and the formation of host cell wall protuberances in intercellular spaces. Hyphae had concentric bodies that showed an electron-transparent core surrounded by an electron-dense layer with radiating filamentous structures on their surface. One or more intrahyphal hyphae were found in the cytoplasm of intercellular or intracellular hyphae. These results suggest that the ligno–suberized cork layers in the wound periderm of citrus act as a protective barrier, which leads to restricted growth of E. fawcettii in bordered scab lesions. The fungus is thought to form concentric bodies and intrahyphal hyphae as a survival mechanism against the water- and nutrient-deficient environments that occur in the cork layers of necrotic host parts.
Similar content being viewed by others
References
Achor DS, Albrigo LG and McCoy CW (1991) Developmental anatomy of lesions on 'Sunburst' mandarin leaves initiated by citrus rust mite feeding. Journal of the American Society for Horticultural Science 116: 663-668
Agrios GN (1997) Plant Pathology, 4th edn, Academic Press, San Diego
Brown RM and Wilson R (1968) Electron microscopy of the lichen Physcia aipolia (Ehrh.) Nyl. Journal of Phycology 4: 230-240
Classen B, Amelunxen F and BlaschekW (2000) Concentric bodies in a parasitic fungus of Malva sylvestris (Malvaceae) pollen. Journal of Phytopathology 148: 313-317
Cunningham HS (1928) Histology of the lesions produced by Sphaceloma fawcettii Jenkins on leaves of citrus. Phytopathology 18: 539-545
Gabel AW and Tiffany LH (1987) Host-parasite relations and development of Elsinoe panici. Mycologia 79: 737-744
Granett AL (1974) Ultrastructural studies of concentric bodies in the ascomycetous fungus Venturia inaequalis. Canadian Journal of Botany 52: 2137-2139
Honegger R (2001) The symbiotic phenotype of lichenforming ascomycetes. In: Hock B (ed) The Mycota. Vol. 9B (pp 165-188) Springer-Verlag, Berlin
Hyun JW, Timmer LW, Lee SC, Yun SH, Ko SW and Kim KS (2001) Pathological characterization and molecular analysis of Elsinoe isolates causing scab diseases of citrus in Jeju Island in Korea. Plant Disease 85: 1013-1017
Jenkins AE (1931) Development of the citrus-scab organism, Sphaceloma fawcettii. Journal of Agricultural Research 42: 545-558
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. Journal of Cell Biology 27: 137A-138A
Kim KW, Park EW, Kim, YH, Ahn, KK, Kim PG and Kim KS (2001) Latency-and defense-related ultrastructural characteristics of apple fruit tissues infected with Botryosphaeria dothidea. Phytopathology 91: 165-172
Kolattukudy PE (1984) Biochemistry and function of cutin and suberin. Canadian Journal of Botany 62: 2918-2933
Lim LL, Fineran, BA and Cole ALJ (1983) Ultrastructure of intrahyphal hyphae of Glomus fasciculatum (Thaxter) Gerdemann and Trappe in roots of white clover (Trifolium repens L.). New Phytologist 95: 231-239
Mason DL and Backus MP (1969) Host-parasite relations in spot anthracnose of Desmodium. Mycologia 61: 1124-1141
Mason DL and Wilson CL (1978) Fine-structure analysis of host-parasite relations in the spot anthracnose of Desmodium. Phytopathology 68: 65-73
Murillo I, Cavallarin L and San Segundo B (1999) Cytology of infection of maize seedlings by Fusarium moniliforme and immunolocalization of the pathogenesis-related PRms protein. Phytopathology 89: 737-747
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. Journal of Cell Biology 17: 208-212
Rushing AE and Latham AJ (1991) Some ultrastructural observations of Cladosporium caryigenum growth in pecan leaves. Phytopathology 81: 1102-1108
Sanders WB and Ascaso C (1997) Fine structural features of rhizomorphs (sensu lato) produced by four species of lichen fungi. Mycological Research 101: 319-328
Scalet M, Crivellato E and Mallardi F (1989) Demonstration of phenolic compounds in plant tissues by an osmium-iodide postfixation procedure. Stain Technology 64: 273-280
Shankar M, Cowling WA and Sweetingham MW (1998) Histological observations of latent infection and tissue colonization by Diaporthe toxica in resistant and susceptible narrow-leafed lupins. Canadian Journal of Botany 76: 1305-1316
Simard M, Rioux D and Laflamme G (2001) Formation of lignosuberized tissues in jack pine resistant to the European race of Gremmeniella abietina. Phytopathology 91: 1128-1140
Sivanesan A and Critchett C(1969) Elsinoe fawcettii. In: Descriptions of Pathogenic Fungi and Bacteria. No. 438 Commonwealth Mycological Institute, Kew
Timmer LW (2000) Scab diseases. In: Timmer LW, Garnsey SM and Graham JH (eds) Compendium of Citrus Diseases, 2nd edn (pp 31-32) APS Press, St. Paul
Whiteside JO (1975) Biological characteristics of Elsinoë fawcettii pertaining to the epidemiology of sour orange scab. Phytopathology 65: 1170-1175
Whiteside JO (1988) Introduction. In: Whiteside JO, Garnsey SM and Timmer LW (eds) Compendium of Citrus Diseases (pp 1-4) APS Press, St. Paul
Williamson B and McNicol RJ (1989) The histology of lesion development in raspberry canes infected by Elsinoe veneta. Annals of Applied Biology 114: 35-44
Zeigler RS, Powell LE and Thurston HD (1980) Gibberellin A4 production by Sphaceloma manihoticola, causal agent of cassava superelongation disease. Phytopathology 70: 589-593
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woo Kim, K., Hyun, JW. & Woo Park, E. Cytology of Cork Layer Formation of Citrus and Limited Growth of Elsinoe fawcettii in Scab Lesions. European Journal of Plant Pathology 110, 129–138 (2004). https://doi.org/10.1023/B:EJPP.0000015330.21280.4c
Issue Date:
DOI: https://doi.org/10.1023/B:EJPP.0000015330.21280.4c