Abstract
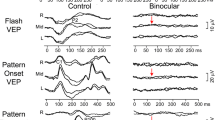
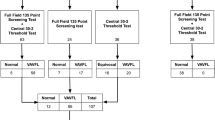
The antiepileptic drug, vigabatrin, has been linked to a specific pattern of visual field loss. The majority of studies have not included the paediatric population due to difficulties assessing visual field function. This is a particular problem as vigabatrin is effective against infantile spasms. A field-specific visual evoked potential was developed which consisted of a central stimulus (0–5° radius) and a peripheral stimulus (30–60° radius). Both stimuli consist of black and white checks which increase in size with eccentricity. Responses are recorded from occipital electrodes O2 and O1 referred to frontal electrode Fz. Electroretinograms and perimetry was performed were possible as a comparison. Thirty-nine children with epilepsy treated with vigabatrin aged from 3 to 15 years were included in the study; 35/39 children complied with the field-specific VEP, 26/39 complied with the ERG and 11/39 performed perimetry. Of these results, 18 children had normal ERG responses and eight had abnormal response. Visual field testing revealed four children had abnormal and seven had abnormal visual field results. The Field-specific VEP identified three of four abnormal perimetry results and six of seven normal perimetry results, giving a sensitivity of 75% and a specificity of 85.7%. When comparing perimetry results with the ERG parameters only the 30-Hz flicker amplitude, with a cut-off amplitude below 70 µV, gave a useful sensitivity of 75% and a specificity of 71%. The field-specific VEP is a useful alternative method that is both well tolerated by young children and gives a reliable indication of likely peripheral visual field loss associated with vigabatrin. The defect appears to have a similar prevalence in children as it does in adults.
Similar content being viewed by others
References
Perucca E. Efficacy and tolerability with long-term administration of vigabatrin. Br J Clin Pract 1988; 42 (Suppl. 61):3.
Appleton RE. Vigabatrin in the management of generalized seizures in children. Seizure1995; 4: 45–8.
Cubells JF, Blanchard JS, Smith DM, Makman MH. In vivo action of enzyme-activated irreversible inhibitors of glutamic acid decarboxylase and ?-aminobutyric acid transaminase in retina vs. brain. J Pharmacol Exp Ther 1986; 238(2): 508–14.
Eke T, Talbot J, Lawden MC. Severe persistent visual field constriction associated with vigabatrin. Br Med J 1997; 314:180–1.
Harding GFA. Severe persistent visual field constriction associated with vigabatrin. Four possible mechanisms exist. Br Med J 1997; 314: 1694.
Krauss GL, Johnson MA, Miller NR. Vigabatrin-associated retinal cone system dysfunction. Electroretinogram Ophthalmol Find Neurol 1998; 50: 814–9.
Arndt CF, Derambure P, Defoort-Dhellemmes S, Hache JC. Outer retinal dysfunction in patients treated with vigabatrin. Neurology 1999; 52: 1201–5.
Daneshvar H, Racette L, Coupland SG, Kertes PJ, Guberman A, Zakon D. Symptomatic and asymptomatic visual loss in patients taking vigabatrin. Ophthalmology 1999; 106: 1792–8.
Kalvianien R, Nousiainen I, Mantyjarvi M, et al. Vigabatrin, a gabaergic antiepileptic drug, causes concentric visual field constriction. Neurology 1999; 53: 922–6.
Lawden MC, Eke T, Degg C, Harding GFA, Wild JM. Visual field defects associated with vigabatrin therapy. J Neurol, Neurosurg Psychiatry 1999; 67: 716–22.
Wild JM, Martinez C, Reinshagen G, Harding GFA. Characteristics of a unique visual field defect attributed to vigabatrin. Epilepsia 1999; 40: 1784–94.
Miller NR, Johnson MA, Paul SR, et al. Visual dysfunction in patients receiving vigabatrin. Clinical and electrophysiological findings. Neurology 1999; 53: 2082–7.
Paul SR, Krauss GL, Miller NR, Medura MT, Miller TA, Johnson MA. Visual function is stable in patients who continue long-term vigabatrin therapy: Implicationsfor clinical decision making. Epilepsia 2001; 42: 525–30.
Malmgren K, Ben-Menachem E, Frisén L. Vigabatrin visual toxicity: Evolution and dose dependence. Epilepsia 2001; 42: 609–15.
Hardus P, Verduin WM, Engelsman M, et al. Visual field loss associated with vigabatrin: Quantification and relation to dosage. Epilepsia 2001; 42: 262–7.
Hammond EJ, Rangel RJ, Wilder BJ. Evoked potential monitoring of vigabatrin patients. Br J Clin Pract 1989; 61: 16–23.
Cosi V, Callieco R, Galimberti CA, et al. Effects of vigabatrin on evoked potentialsin epileptic patients. Br J Clin Pharmacol 1989; 27: 61–8S.
Liegeois-Chauvel C, Marquis P, Gisselbrecht D, Pantieri R, Beaumont D, Chauvel P. Effectsof long-term vigabatrin on somatosensory-evoked potentials in epileptic patients. Epilepsia 1989; 30: S23–5.
Harding GFA, Jones LA, Tipper VJ, Betts TA, Mumford JP. Electroretinogram, pattern electroretinogram and visual evoked potential assessment in patients receiving vigabatrin. Epilepsia 1995; 36: S108.
Mauguiére F, Chauvel P, Dewailly J, Dousse N. No Effect of long-term vigabatrin treatment on CNS conduction in epileptic patients: results of a multicenter study of somatosensory and visual evoked potentials. Epilepsia 1995; 36: 29.
Harding GFA, Robertson KA, Edson AS, Barnes P, Wild JM. Visual electrophysiological effect of a GABA transaminase blocker. Doc Ophthalmol. 1999; 97: 179–88.
Harding GFA, Wild JM, Robertson KA, Rietbrock S, Martinez C. Separating the retinal electrophysiologic effects of vigabatrin - treatment versus field loss. Neurology 2000; 55(3): 347–52.
Harding GFA, Wild JM, Robertson K, et al. EOGs, ERGs, VEPs and multifocal ERGs in epileptic patients showing visual field constriction. Epilepsia 2000; 41(11): 1420–31.
Chiron C, Dulac O, Beaumont D, Palacios L, Pajot N, Mumford J. Therapeutic trial of vigabatrin in refactory infantile spasms. J Child Neurol 1991; 6: 2S52–9.
Harding GFA, Robertson K, Holliday I, Jones L. Field-specific visual evoked potentials for assessment of peripheral field defect in a paediatric population. J Physiol 1999; 518P: 171P.
Harding GFA, Robertson KA, Holliday I. Field specific visual evoked potentials for assessment of peripheral field defect in a paediatric population. In: Ambler Z, Nevþímalová S, Kadaóka Z, Rossini PM, eds. Clinical neurophysiology at the beginning of the 21st century. Supplements to Clin Neurophysiol 2000; 53: 323–30.
Harding GFA, Spencer EL, Wild JM, Conway M, Bohn, RL. Field-specific visual evoked potentials: Identifying field defectsin Vigabatrin-treated children. Neurology 2002; 58:1261–5.
Marmor MF, Zrenner E. Standard for clinical electroretinography. Doc Ophthalmol 1998; 97: 143–56.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spencer, E., Harding, G. Examining visual field defects in the paediatric population exposed to vigabatrin. Doc Ophthalmol 107, 281–287 (2003). https://doi.org/10.1023/B:DOOP.0000005337.39947.83
Issue Date:
DOI: https://doi.org/10.1023/B:DOOP.0000005337.39947.83