Abstract
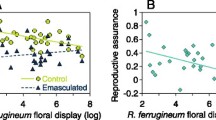
Genetic factors influence the populationviability of rare species, yet the fitnessconsequences of inbred and outbred progeny areseldom tested empirically in reintroductionstrategies designed for species recovery orhabitat restoration. Rare and endangeredplants of Silene (Caryophyllaceae) occuron four continents, including North America. In Oregon, inbred and outbred progeny weremonitored for three years after experimentalreintroduction of a narrow endemic, S.douglasii var. oraria, into formerlygrazed habitat within its presumed historicalrange. Survival and reproduction were comparedfor progeny that were derived from the seeds ofself- versus cross-pollinated flowersproduced in situ at Cascade Head, aUNESCO Biosphere Reserve where the largest ofthree extant populations occurs. Progeny ofcross-pollinated flowers had significantlygreater field survival in all years than didoffspring of selfed or open-pollinated flowers(P < 0.01). Outbred progeny alsosignificantly exceeded other treatment cohortsin canopy area, and produced more reproductivestems and flowers than other progeny types ofthe same maternity. For plots varying in plantdensity, mortality was greatest in thehigh-density competitive regime but thesurvivors reached significantly larger sizesand reproductive capacities than in low densityplots (P < 0.05). In all, successfulconservation plans involving reintroduction mayrequire genetically diverse progeny to offsetinbreeding depression as well as suitableplanting densities and source populations.
Similar content being viewed by others
References
Anderson R, Baskin J (1999) Savannas, Barrens, and Rock Outcrop Plant Communities of North America.Cambridge University Press, Cambridge.
Antonovics J, Thrall P, Jarosz A, Stratton D (1994) Ecological genetics of metapopulations: Silene-Ustilagoplant-pathogen system. In: Ecological Genetics (ed. Real L), pp. 146–170. Princeton University Press, Princeton.
Barrett S, Kohn J (1991) Genetic and evolutionary consequences of small population size in plants: implications for conservation. In: Conservation of Rare Plants (eds. Falk D, Holsinger K), pp. 3–30. Oxford University Press, Oxford.
Barbour M, Major J (1995) Terrestrial Vegetation of California.Special publication 9. California Native Plant Society, Sacramento.
Brown E, Kephart S (1999) Variability in pollen load: Implications for reproduction and seedling vigor in a rare plant, Silene douglasiivar. oraria. Int. J. of Plant Sci., 160, 1145–1152.
Bowles, M, McBride J, Betz R (1998) Management and restoration ecology of the federal threatened Mead's milkweed, Asclepias meadii(Asclepiadaceae). Ann. Mo. Bot. Gard., 85, 110–125.
Byers D, Waller D (1999) Do plant populations purge their genetic load? Effects of population size and mating history on inbreeding depression. Annu. Rev. Ecol. Sys., 30, 479–513.
Charlesworth B, Charlesworth D, Morgan M (1990) Genetic loads and estimates of mutation rates in highly inbred populations. Nature, 347, 380–382.
Dolan R (1994) Patterns of isozyme variation in relation to population size, isolation, and phytogeographic history in royal catchfly (Silene regia). Amer. J. Bot., 81, 965–972.
Dudash M (1990) Relative fitness of selfed and outcrossed progeny in a self-compatible, protandrous species, Sabatia angularisL. (Gentianaceae): A comparison in three environments. Evolution, 44, 1129–1139.
Dudash M, Fenster C (2001) The role of breeding system and inbreeding depression in the maintenance of an outcrossing mating strategy in Silene virginica(Caryophyllaceae). Amer. J. Bot., 88, 1953–1959.
Ebert D, Haag C, Kirkpatrick M, Riek M, Hottinger J, Pajunen V (2002) A selective advantage to immigrant genes in a Daphniametapopulation. Science, 295, 485–488.
Ellstrand N, Elam D (1993) Population genetic consequences of small population size: Implications for plant conservation. Annu. Rev. Ecol. Sys., 24, 217–242.
Federal Register (1993, 1996) Plant Taxa for Listing as Endangered or Threatened Species. Department of the Interior, Fish and Wildlife Service, USA.
Fenster C, Dudash M (1994) Genetic considerations for plant population restoration and conservation. In: Restoration of Endangered Species (eds. Bowles M, Whelan C), pp. 34–62. Cambridge University Press, Cambridge.
Fenster C, Galloway L (2000) Inbreeding and outbreeding depression in natural populations of Chamaecrista fasciculata(Fabaceae). Conserv. Biol., 14, 1406–1412.
Fiedler P, Knapp B, Fredricks N (1998) Rare plant demography: Lessons from the mariposa lilies (Calochortus: Liliaceae). In: Conservation Biology (eds. Fiedler P, Kareva P), pp. 28–48. Chapman & Hall, New York.
Frankham R, Gilligan D, Morris D, Briscoe D (2001) Inbreeding and extinction: Effects of purging. Conserv. Genet., 2, 279–285.
Friar E, Boose D, LaDoux T, Roalson E, Robichaux H (2001) Population structure in the endangered Mauna Loa silversword, Argyroxiphium kauense(Asteraceae), and its bearing on reintroduction. Mol. Ecol. 10, 1657–1663.
Giles B, Goudet J (1997) Genetic differentiation in Silene dioicametapopulations: Estimation of spatio-temporal effects in a successional plant species. Am. Nat., 149, 507–526.
Guerrant E (1996) Designing populations: Demographic, genetic, and horticultural dimensions. In: Restoring Diversity (eds. Falk D, Millar C, Olwell M), pp. 171–208. Island Press, Washington D.C.
Haikola S (2001) Inbreeding depression and the maintenance of genetic load in Melitaea cinxiametapopulations. Conserv. Genet., 2, 325–335.
Hanski I, Gilpin M (1997) Metapopulation dynamics: brief history and conceptual domain. Biol. J. Linn. Soc., 42, 3–16.
Hedrick P, Kalinowski S (2000) Inbreeding depression in conservation biology. Annu. Rev. Ecol. Sys., 31, 139–162.
Helenurm K, Parsons L (1997) Genetic variation and the reintroduction of Cordylanthus maritimusssp maritimusto Sweetwater Marsh, California. Restor. Ecol., 5, 236–244.
Helenurm K (1998) Outplanting and differential source population success in Lupinus guadallupensis. Conserv. Biol., 12, 118–127.
Herschel S, Paige K (1995) Inbreeding depression, environmental stress, and population size variation in Scarlet gilia (Imposis aggregata). Conserv. Biol., 9, 126–133.
Holsinger K (2000) Demography and extinction in small populations. In: Genetics, Demography and Viability of Fragmented Populations (eds. Young A, Clarke G), pp. 55–74. Cambridge University Press, Cambridge.
Invarsson P, Giles B (1999) Kin-structured colonization and smallscale genetic differentiation in Silene dioica. Evolution, 53, 605–611.
Jiménez J, Hughes K, Alaks G, Graham L, Lacy R (1994) An experimental study of inbreeding depression in a natural habitat. Science, 266, 271–273.
Kephart S, Paladino C (1997) Demographic change and microhabitat variability in a grassland endemic, Silene douglasiivar. oraria(Caryophyllaceae). Amer. J. Bot., 84, 179–189.
Kephart S, Sturgeon K, Lum J, Bledsoe K (1999a) Varietal relationships in Silene douglasii(Caryophyllaceae): morphological variability at the population level. Sys. Bot., 24, 529–544.
Kephart S, Brown E, Hall J (1999b) Inbreeding depression and partial selfing: Evolutionary implications of mixed-mating in a coastal endemic, Silene douglasiivar. oraria(Caryophyllaceae). Heredity, 82, 543–554.
Knapp E, Dyer A (1998) When do genetic considerations require special approaches to ecological restoration? In: Conservation Biology (eds. Fiedler P, Kareva P), pp. 345–363. Chapman & Hall, New York.
Lesica P (1997) Demography of the endangered plant. Silene spaldingii(Caryophyllaceae) in northwest Montana. Madroño, 40, 193–201.
Lesica P, Allendorf F (1992) Are small populations of plants worth preserving? Conserv. Biol., 6, 135–139.
Levins R (1969) Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull. Entomol. Soc. Amer., 15, 137–240.
Luijten S, Marckéry J, Oostermeijer J, den Nijs J (2002) Demographic consequences of inbreeding and outbreeding in Arnica montana: A field experiment. J. Ecol., 90, 592–603.
Martins P, Jain S (1979) Role of genetic variation in the colonizing ability of rose clover (Trifolium hirtumAll.) Am. Nat., 114, 591–595.
Maunder M (1992) Plant reintroduction: An overview. Biodivers. Conserv., 1, 51–61.
McCauley D, Raveill J, Antonovics J (1995) Local founding events as determinants of genetic structure in a plant metapopulation. Heredity, 75, 630–636.
McCauley D, Richards D, Emery S, Smith R, McGlothlin (2001) The interaction of genetic and demographic processes in plant metapopulation. In: Integrating Ecology and Evolution in a Spatial Context (eds. Silvertown J, Antonovics J), pp. 177–196. Blackwell Science Inc., London.
McGlaughlin M, Karoly K, Kaye T (2002) Genetic variation and its relationship to population size in reintroduced populations of the pink sand verbena, Abronia umbellatasubsp. breviflora(Nyctaginaceae). Conserv. Genet., 3, 411–420.
McKinney M (1997) Extinction vulnerability and selectivity: Combining ecological and paleontological views. Annu. Rev. Ecol. Sys., 28, 495–516.
Menges E (1992) Stochastic modeling of extinction in plant populations. In: Conservation Biology. The Theory and Practice of Nature Conservation, Preservation and Management (eds. Fiedler P, Jain S), pp. 254–275. Chapman & Hall, New York.
Menges E, Dolan R (1998) Demographic viability of populations of Silene regiain midwestern prairies: Relationships with fire management, genetic variation, geographic location, population size and isolation. J. Ecol., 86, 63–78.
Montalvo A, Williams S, Rice K, Buchmann S, Cory C, Handel S, Nabhan G, Primack R, Robichaux R (1997) Restoration biology: A population biology perspective. Restor. Ecol., 5, 277–290.
Morgan J (2000) Reproductive success in reestablished versus natural populations of a threatened grassland daisy (Rutidosis leptorrhynchoides). Conserv. Biol., 14, 780–785.
Newman D, Pilson, D (1997) Increased probability of extinction due to decreased genetic effective population size: Experimental populations of Clarkia pulchella. Evolution, 51, 354–362.
Oostermeijer JG, van Eijck M, den Nijs H (1994) Offspring fitness in relation to population size and genetic variation in the rare perennial plant species Gentiana pneumonanthe(Gentianaceae). Oecologia 97, 289–296.
Oostermeijer JG (2000) Demography species Gentiana pneumonanthe: the importance of genetics, demography, and reproductive biology. In: Genetics, Demography and Viability of Fragmented Populations (eds. Young A, Clarke G), pp. 55–74. Cambridge University Press, New York.
Pavlik B, Manning E (1993) Assessing limitations on the growth of endangered plant populations, I. Experimental demography of Erysimum capitatumssp. angustatumand Oenothera deltoidesssp. Howellii. Biol. Conserv., 65, 257–265.
Pavlik B, Nickrent D, Howald A (1993) The recovery of an endangered plant, I. Creating a new population of Amsinckia grandiflora. Conserv. Biol., 7, 510–526.
Polans N, Allard R (1989) An experimental evaluation of the recovery potential of rye grass populations from genetic stress resulting from restriction of population size. Evolution, 43, 1320–1324.
Rankin A, Weller S, Sakai A (2002) Mating system instability in Schiedia menziesii(Caryophyllaceae). Evolution, 56, 1574–1585.
Richards C (2000a) Genetic and demographic influences on population persistence: Gene flow and genetic rescue in Silene alba.In: Genetics, Demography and Viability of Fragmented Populations (eds. Young A, Clarke G), pp. 271–291. Cambridge University Press, New York.
Richards C (2000b) Inbreeding depression and genetic rescue in a plant metapopulation. Am Nat., 155, 383–394.
Ripley J (1983) Description of the Plant Communities and Sucession of the Oregon Coast Grasslands. Ph.D Thesiis, Oreg State University, Corvallis.
Saccheri M, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I (1997) Inbreeding and extinction in a butterfly metapopulation. Nature, 392, 491–494.
Schemske D, Husband B, Ruckelshaus M, Goodwillie C, Parker I, Bishop G (1994) Evaluating apporaches to the conservation of rare and endangered plants. Ecology, 75, 584–606.
Schoen D, Brown A (2001) The conservation of wild plant species in seed banks. BioScience, 51, 960–966.
Schwartz M (1999) Choosing the appropriate scale of reserves for conservation. Annu. Rev. Ecol. Sys., 30, 83–108.
Sheridan M, Karowe D (2000) Inbreeding, outbeeding, and heterosis in the yellow pitcher plant, Sarracenia flava (Sarraceniacea), in Virginia. Amer. J. Bot., 87, 1628–1633.
Sherwin W, Moritz C (2000) Managing and monitoring genetic erosion. In: Genetics, Demography and Viability of Fragmented Populations (eds. Young A, Clarke G), pp. 9–34. Cambridge University Press.
Smulders M, van der Schoot J, Geerts R, Antoinisse-de Jong A, Korevaar H, van der Werf A, Vosman B (2000) Genetic diversity and the reintroduction of meadow species. Plant Biol., 2, 447–454.
Soule M, Mills S (1998) No need to isolate genetics. Science, 282, 1658–1659.
Stromberg M, Kephart P, Yadon V (2001) Composition, invasibility, and diversity in coastal California grasslands. Madroño, 48, 236–252.
Stüwe M, Scribner K (1989) Low genetic variability in reintroduced alpine ibex (Capra ibex ibex) populations. J. Mammol., 70, 370–373.
Tilman D, Meej R, Lehman C, Nowak M (1994) Habitat destruction and the extinction debt. Nature, 371, 65–66.
Walter K, Gillett H (1998) IUCN Red List of Threatened Plants. Cambridge, UK.
Waser N, Price M (1994) Crossing-distance effects in Delphinium nelsonii(Ranunculaceae): Outbreeding and inbreeding depression in progeny fitness. Evolution, 48, 842–852.
Westerbergh A, Saura A (1994) Gene flow and pollinator behaviour in Silene dioicapopulations. Oikos, 71, 215–224.
Williams S, Davis C (1996) Population genetic analyses of transplanted eelgrass (Zostera marina) beds reveal reduced genetic diversity in southern California. Restor. Ecol., 4, 163–180.
Williamson P, Werth C (1999) Levels and patterns of genetic variation in the endangered species Abronia macrocarpa(Nyctaginaceae). Amer. J. Bot., 86, 293–301.
Willis J (1999) Inbreeding load, average dominance and the mutation rate for midly deleterious alleles in Mimulus guttatus. Evolution, 53, 1678–1691.
Wolfe A, Xiang Q, Kephart S (1998) Assessing hybridization in natural populations of Penstemon(Scrophulariaceae) using hypervariable inter-simple sequence repeat (ISSR) bands. Mol. Ecol., 7, 1107–1125.
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trn. Ecol. Evol., 11, 413–417.
Young A, Murray A (2000) Genetic bottlenecks and dysgenic flow into re-established populations of the grassland daisy, Rutidosis leptorrhynchoides. Austr. J. Bot., 48, 409–416.
Zar, J (1996) Biostatistical Analysis. Prentice Hall, New Jersey.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kephart, S.R. Inbreeding and reintroduction: Progeny success in rare Silene populations of varied density. Conservation Genetics 5, 49–61 (2004). https://doi.org/10.1023/B:COGE.0000014056.65197.c4
Issue Date:
DOI: https://doi.org/10.1023/B:COGE.0000014056.65197.c4