Abstract
1. This study aims (1) to determine whether secretin is synthesized centrally, specifically by the HPA axis and (2) to discuss, on the basis of the findings in this and previous studies, secretin's possible neuroregulatory role in autism.
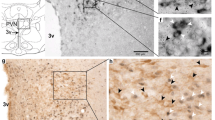
2. An immunocytochemical technique with single-cell resolution was performed in 12 age/weight-matched male rats pretreated with stereotaxic microinjection of colchicine (0.6 μg/kg) or vehicle into the lateral ventricle. Following 2-day survival, rats were anesthetized and perfused for immunocytochemistry. Brain segments were blocked and alternate frozen 30-μm sections incubated in rabbit antibodies against secretin, vasoactive intestinal peptide, glucagon, or pituitary-adenylate-cyclase-activating peptide. Adjacent sections were processed for Nissl stain. Preadsorption studies were performed with members of the secretin peptide family to demonstrate primary antibody specificity.
3. Specificity of secretin immunoreactivity (ir) was verified by clear-cut preadsorption control data and relatively high concentrations and distinct topographic localization of secretin ir to paraventricular/supraoptic and intercalated hypothalamic nuclei. Secretin levels were upregulated by colchicine, an exemplar of homeostatic stressors, as compared with low constitutive expression in untreated rats.
4. This study provides the first direct immunocytochemical demonstration of secretinergic immunoreactivity in the forebrain and offers evidence that the hypothalamus, like the gut, is capable of synthesizing secretin. Secretin's dual expression by gut and brain secretin cells, as well as its overlapping central distribution with other stress-adaptation neurohormones, especially oxytocin, indicates that it is stress-sensitive. A neuroregulatory relationship between the peripheral and central stress response systems is suggested, as is a dual role for secretin in conditioning both of those stress-adaptation systems. Colchicine-induced upregulation of secretin indicates that secretin may be synthesized on demand in response to stress, a possible mechanism of action that may underlie secretin's role in autism.
Similar content being viewed by others
REFERENCES
Agassandian, K., Fazan, V. P., Adanina, V., and Talman W. T. (2002). Direct projections from the cardiovascular nucleus tractus solitarii to pontine preganglionic parasympathetic neurons: A link to cerebrovascular regulation. J. Comp. Neurol. 452(3): 242-254.
Aguado, F., Pozas, E., and Blasi, J. (1999). Colchicine administration in the rat central nervous system induces SNAP-25 expression. Neuroscience 93(1): 275-283.
Anisman, H., Zaharia, M. D., Meaney, M. J., and Merali, Z. (1998). Do early-life events permanently alter behavioral and hormonal responses to stressors? Int. J. Dev. Neurosci. 16(3/4): 149-164.
Armando, I., Carranza, A., Nishimura, Y., Hoe, K. L., Barontini, M., Terron, J. A., Falcon-Neri, A., Ito, T., Juorio, A. V., and Saavedra, J. M. (2001). Peripheral administration of an angiotensin II AT(1) receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation stress. Endocrinology 142(9): 3880-3889.
Barlow, B., and Santulli, T. V. (1975). Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery 77(5): 687-690.
Bauman, M., and Kemper, T. L. (1985). Histoanatomic observations of the brain in early infantile autism. Neurology 35(6): 866-874.
Bayliss, W. M., and Starling, E. H. (1902). The mechanism of pancreatic secretion. J. Physiol. (Lond.) 28: 325-353.
Bell, P. M., Henry, R. W., Buchanan, K. D., and Alberti, K. G. (1984). The effect of starvation on the gastro-entero-pancreatic hormonal and metabolic responses to exercise. (GEP hormones in starvation and exercise). Diabete Metab. 10(3): 194-198.
Bloom, S. R., Mitchell, S. J., Greenberg, G. R., Christofides, N., Domschke, W., Domschke, S., Mitznegg, P., and Demling, L. (1978). Release of VIP, secretin and motilin after duodenal acidification in man. Acta Hepatogastroenterol. (Stuttg.) 25(5): 365-368.
Boccia, D., Stolfi, I., Lana, S., and Moro, M. L. (2001). Nosocomial necrotising enterocolitis outbreaks: epidemiology and control measures. Eur. J. Pediatr. 160(6): 385-391.
Bojanowska, E., Juszczak, M., Guzek, J. W., and Dabrowski, R. (1999). The pineal and oxytocin synthesis. J. Physiol. Pharmacol. 50(1): 121-128.
Booth, R., Charlton, R., Hughes, C., and Happe, F. (2003). Disentangling weak coherence and executive dysfunction: Planning drawing in autism and attention-deficit/hyperactivity disorder. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358(1430): 387-392.
Bredy, T. W., Humpartzoomian, R. A., Cain, D. P., and Meaney, M. J. (2003). Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience 118(2): 571-576.
Bregonzio, C., Armando, I., Ando, H., Jezova, M., Baiardi, G., and Saavedra, J. M. (2003). Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am. J. Physiol. Gastrointest. Liver Physiol. 285(2): G414-G423.
Breiner, J., and Beck, S. (1984). Parents as change agents in the management of their developmentally delayed children's noncompliant behaviors: A critical review. Appl. Res. Ment. Retard. 5(2): 259-278.
Chang, T. M., Berger-Ornstein, L., and Chey, W. Y. (1985). Presence of biologically and immunologically active secretin-like substance in the mammalian brain. Peptides 6(2): 193-198.
Chang, T. M., Chang, C. H., Wagner, D. R., and Chey, W. Y. (1999). Porcine pancreatic phospholipase A2 stimulates secretin release from secretin-producing cells. J. Biol. Chem. 274(16): 10758-10764.
Charlton, C. G., Miller, R. L., Crawley, J. N., Handelmann, G. E., and O'Donohue, T. L. (1983). Secretin modulation of behavioral and physiological functions in the rat. Peptides 4(5): 739-742.
Charlton, C. G., O'Donohue, T. L., Miller, R. L., Jacobowitz, D. M. (1981). Secretin immunoreactivity in rat and pig brain. Peptides 2(Suppl. 1): 45-49.
Chey, W. Y., and Chang, T. (2001). Neural hormonal regulation of exocrine pancreatic secretion. Pancreatology 1(4): 320-335.
Chriguer, R. S., Rocha, M. J., Antunes-Rodrigues, J., and Franci, C. R. (2001). Hypothalamic atrial natriuretic peptide and secretion of oxytocin. Brain Res. 889 (1/2): 239-242.
Chugani, D. C., Sundram, B. S., Behen, M., Lee, M. L., and Moore, G. J. (1999). Evidence of altered energy metabolism in autistic children. Prog. Neuropsychopharmacol. Biol. Psychiatry 23(4): 635-641.
Ciriello, J., Rosas-Arellano, M. P., Solano-Flores, L. P., de Oliveira, C. V. (2003). Identification of neurons containing orexin-B (hypocretin-2) immunoreactivity in limbic structures. Brain Res. 967 (1/2): 123-131.
Coniglio, S. J., Lewis, J. D., Lang, C., Burns, T. G., Subhani-Siddique, R., Weintraub, A., Schub, H., and Holden, E. W. (2001). A randomized, double-blind, placebo-controlled trial of single-dose intravenous secretin as treatment for children with autism. J. Pediatr. 138(5): 649-655.
Corona, R., Dissanayake, C., Arbelle, S., Wellington, P., and Sigman, M. (1998). Is affect aversive to young children with autism? Behavioral and cardiac responses to experimenter distress. Child Dev. 69(6): 1494-1502.
Davis, E., Fennoy, I., Laraque, D., Kanem, N., Brown, G., and Mitchell, J. (1992). Autism and developmental abnormalities in children with perinatal cocaine exposure. J. Natl. Med. Assoc. 84(4): 315-319.
de Lecea, L., Kilduff, T. S., Peyron, C., Gao, X., Foye, P. E., Danielson, P. E., Fukuhara, C., Battenberg, E. L., Gautvik, V. T., Bartlett, F. S., II, Frankel, W. N., van den Pol, A. N., Bloom, F. E., Gautvik, K. M., and Sutcliffe, J. G. (1998). The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U.S.A. 95(1): 322-327.
D'Eufemia, P., Celli, M., Finocchiaro, R., Pacifico, L., Viozzi, L., Zaccagnini, M., Cardi, E., and Giardini, O. (1996). Abnormal intestinal permeability in children with autism. Acta Paediatr. 85(9): 1076-1079.
Diggle, T., McConachie, H. R., and Randle, V. R. (2003). Parent-mediated early intervention for young children with autism spectrum disorder. Cochrane Database Syst. Rev. (1): CD003496.
Dunn-Geier, J., Ho, H. H., Auersperg, E., Doyle, D., Eaves, L., Matsuba, C., Orrbine, E., Pham, B., and Whiting, S. (2000). Effect of secretin on children with autism: A randomized controlled trial. Dev. Med. Child Neurol. 42(12): 796-802.
Eriksson, M., Bjorkstrand, E., Smedh, U., Alster, P., Matthiesen, A. S., and Uvnas-Moberg, K. (1994). Role of vagal nerve activity during suckling. Effects on plasma levels of oxytocin, prolactin, VIP, somatostatin, insulin, glucagon, glucose and of milk secretion in lactating rats. Acta Physiol. Scand. 151(4): 453-459.
Francis, D. D., Young, L. J., Meaney, M. J., and Insel, T. R. (2002). Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. J. Neuroendocrinol. 14(5): 349-353.
Freeman, J. H., Jr., Weible, A., Rossi, J., and Gabriel, M. (1997). Lesions of the entorhinal cortex disrupt behavioral and neuronal responses to context change during extinction of discriminative avoidance behavior. Exp. Brain Res. 115(3): 445-457.
Fremeau, R. T., Jr., Korman, L. Y., and Moody, T. W. (1986). Secretin stimulates cyclic AMP formation in the rat brain. J. Neurochem. 46(6): 1947-1955.
Friedman, G. B., Taylor, C. T., Parkos, C. A., and Colgan, S. P. (1998). Epithelial permeability induced by neutrophil transmigration is potentiated by hypoxia: Role of intracellular cAMP. J. Cell Physiol. 176(1): 76-84.
Fuxe, K., Andersson, K., Hokfelt, T., Mutt, V., Ferland, L., Agnati, L. F., Ganten, D., Said, S., Eneroth, P., and Gustafsson, J. A. (1979). Localization and possible function of peptidergic neurons and their interactions with central catecholamine neurons, and the central actions of gut hormones. Fed. Proc. 38(9): 2333-2340.
Gabry, K. E., Chrousos, G. P., Rice, K. C., Mostafa, R. M., Sternberg, E., Negrao, A. B., Webster, E. L., McCann, S. M., and Gold, P. W. (2002). Marked suppression of gastric ulcerogenesis and intestinal responses to stress by a novel class of drugs. Mol. Psychiatry 7(5): 474-483.
Gandhi, S., Tsueshita, T., Onyuksel, H., Chandiwala, R., and Rubinstein, I. (2002). Interactions of human secretin with sterically stabilized phospholipid micelles amplify peptide-induced vasodilation in vivo. Peptides 23(8): 1433-1439.
Gauthier, A., Puente, J. L., and Finlay, B. B. (2003). Secretin of the enteropathogenic Escherichia coli type III secretion system requires components of the type III apparatus for assembly and localization. Infect Immun. 71(6): 3310-3319.
Gershon, M. D. (1998). The Second Brain: A Groundbreaking New Understanding of Nervous Disorders of the Stomach and Intestine, Harper Collins, New York.
Golanov, E. V., Ruggiero, D. A., and Reis, D. J. (2000). A brainstem area mediating cerebrovascular and EEG responses to hypoxic excitation of rostral ventrolateral medulla in rat. J. Physiol. 529(Pt. 2): 413-429.
Goulet, M., Shiromani, P. J., Ware, C. M., Strong, R. A., Boismenu, R., Rusche, J. R. (2003). A secretin i.v. infusion activates gene expression in the central amygdala of rats. Neuroscience 118(4): 881-888.
Graveling, R. A., and Brooke, J. D. (1978). Hormonal and cardiac response of autistic children to changes in environmental stimulation. J. Autism Child Schizophr. 8(4): 441-455.
Guilloteau, P., Chayvialle, J. A., Toullec, R., Grongnet, J. F., and Bernard, C. (1992). Early-life patterns of plasma gut regulatory peptide levels in calves: Effects of the first meals. Biol. Neonate. 61(2): 103-109.
Harmar, A. J. (2001). Family-B G-protein-coupled receptors. Genome Biol. 2(12): Reviews 3013.
Haznedar, M. M., Buchsbaum, M. S., Wei, T. C., Hof, P. R., Cartwright, C., Bienstock, C. A., and Hollander, E. (2000). Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am. J. Psychiatry 157(12): 1994-2001.
Helou, C. M., Imbert-Teboul, M., Doucet, A., Rajerison, R., Chollet, C., Alhenc-Gelas, F., and Marchetti, J. (2003). Angiotensin receptor subtypes in thin and muscular juxtamedullary efferent arterioles of rat kidney. Am. J. Physiol. Renal. Physiol. 285(3): F507-F514.
Hollander, E., King, A., Delaney, K., Smith, C. J., and Silverman, J. M. (2003a). Obsessive-compulsive behaviors in parents of multiplex autism families. Psychiatry Res. 117(1): 11-16.
Hollander, E., Novotny, S., Hanratty, M., Yaffe, R., DeCaria, C. M., Aronowitz, B. R., and Mosovich, S. (2003b). Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology 28(1): 193-198.
Horvath, K., Papadimitriou, J. C., Rabsztyn, A., Drachenberg, C., and Tildon, J. T. (1999). Gastrointestinal abnormalities in children with autistic disorder. J. Pediatr. 135(5): 559-563.
Horvath, K., and Perman, J. A. (2002). Autism and gastrointestinal symptoms. Curr. Gastroenterol Rep. 4(3): 251-258.
Horvath, K., Stefanatos, G., Sokolski, K. N., Wachtel, R., Nabors, L., and Tildon, J. T. (1998). Improved social and language skills after secretin administration in patients with autistic spectrum disorders. J. Assoc. Acad. Minor. Phys. 9(1): 9-15.
Houben, H., and Denef, C. (1994). Bioactive peptides in anterior pituitary cells. Peptides 15(3): 547-582.
Ichihara, K., Eng, J., and Yalow, R. S. (1983). Ontogeny of immunoreactive CCK, VIP and secretin in rat brain and gut. Biochem. Biophys. Res. Commun. 112(3): 891-898.
Iqbal Z. (2002). Ethical issues involved in the implementation of a differential reinforcement of inappropriate behaviour programme for the treatment of social isolation and ritualistic behaviour in an individual with intellectual disabilities. J. Intellect. Disabil. Res. 46(Pt. 1): 82-93.
Itoh, N., Furuya, T., Ozaki, K., Ohta, M., and Kawasaki, T. (1991). The secretin precursor gene. Structure of the coding region and expression in the brain. J. Biol. Chem. 266(19): 12595-12598.
Jezova, M., Armando, I., Bregonzio, C., Yu, Z. X., Qian, S., Ferrans, V. J., Imboden, H., and Saavedra, J. M. (2003). Angiotensin II AT(1) and AT(2) receptors contribute to maintain basal adrenomedullary norepinephrine synthesis and tyrosine hydroxylase transcription. Endocrinology 144(5): 2092-tt22101.
Jin, H. O., Lee, K. Y., Chang, T. M., Chey, W. Y., and Dubois, A. (1994). Secretin: A physiological regulator of gastric emptying and acid output in dogs. Am. J. Physiol. 267(4, Pt. 1): 702-708.
Jones, D., and Gonzalez-Lima, F. (2001). Mapping Pavlovian conditioning effects on the brain: Blocking, contiguity, and excitatory effects. J. Neurophysiol. 86(2): 809-823.
Jones, G. R., Singer, P. P., and Bannach, B. (2002). Application of LC-MS analysis to a colchicine fatality. J. Anal. Toxicol. 26(6): 365-369.
Kern, J. K., Van Miller, S., Evans, P. A., and Trivedi, M. H. (2002). Efficacy of porcine secretin in children with autism and pervasive developmental disorder. J. Autism Dev. Disord. 32(3): 153-160.
Kobayashi, R., Takenoshita, Y., Kobayashi, H., Kamijo, A., Funaba, K., and Takarabe, M. (2001). Early intervention for infants with autistic spectrum disorders in Japan. Pediatr. Int. 43(2): 202-208.
Kopin, A. S., Wheeler, M. B., and Leiter, A. B. (1990). Secretin: Structure of the precursor and tissue distribution of the mRNA. Proc. Natl. Acad. Sci. U.S.A. 87(6): 2299-2303.
Koren, G. (2001). Repeated doses of porcine secretin in the treatment of autism: A randomized, placebo-controlled trial. Pediatrics 107(5): E71.
Koves, K., Kausz, M., Reser, D., and Horvath, K. (2002). What may be the anatomical basis that secretin can improve the mental functions in autism? Regul. Pept. 109(1-3): 167-172.
Lamson, D. W., and Plaza, S. M. (2001). Transdermal secretin for autism—A case report. Altern. Med. Rev. 6(3): 311-313.
Langworthy-Lam, K. S., Aman, M. G., and Van Bourgondien, M. E. (2002). Prevalence and patterns of use of psychoactive medicines in individuals with autism in the Autism Society of North Carolina. J. Child Adolesc. Psychopharmacol. 12(4): 311-321.
Lauterbach, H. H., Lehmann, E., Escobar-Jimenez, F., Mattes, P., and Raptis, S. (1980). Behavior of secretin in the hypoxia-immobilisation stress on the rat. Eur. Surg. Res. 12(2): 103-107.
Leong, D. S., Terron, J. A., Falcon-Neri, A., Armando, I., Ito, T., Johren, O., Tonelli, L. H., Hoe, K. L., and Saavedra, J. M. (2002). Restraint stress modulates brain, pituitary and adrenal expression of angiotensin II AT(1A), AT(1B) and AT(2) receptors. Neuroendocrinology 75(4): 227-240.
Li, P., Chang, T. M., and Chey, W. Y. (1998). Secretin inhibits gastric acid secretion via a vagal afferent pathway in rats. Am. J. Physiol. 275(1, Pt. 1): G22-G28.
Li, Y. W., and Guyenet, P. G. (1996). Angiotensin II decreases a resting K + conductance in rat bulbospinal neurons of the C1 area. Circ. Res. 78(2): 274-282.
Lightdale, J. R., Hayer, C., Duer, A., Lind-White, C., Jenkins, S., Siegel, B., Elliott, G. R., and Heyman, M. B. (2001). Effects of intravenous secretin on language and behavior of children with autism and gastrointestinal symptoms: A single-blinded, open-label pilot study. Pediatrics 108(5): 90.
Loewy, A. D. (1991). Forebrain nuclei involved in autonomic control. Prog. Brain Res. 87: 253-268.
Lopez, M. J., Upchurch, B. H., Rindi, G., and Leiter, A. B. (1995). Studies in transgenic mice reveal potential relationships between secretin-producing cells and other endocrine cell types. J. Biol. Chem. 270(2): 885-891.
Lucas, A., Adrian, T. E., Bloom, S. R., Aynsley-Green, A. (1980). Plasma secretin in neonates. Acta Paediatr. Scand. 69(2): 205-210.
Lucas, A., Bloom, S. R., and Aynsley-Green, A. (1983). Metabolic and endocrine consequences of depriving preterm infants of enteral nutrition. Acta Paediatr. Scand. 72(2): 245-249.
Matthiesen, A. S., Ransjo-Arvidson, A. B., Nissen, E., and Uvnas-Moberg, K. (2001). Postpartum maternal oxytocin release by newborns: Effects of infant hand massage and sucking. Birth 28(1): 13-19.
McEwen, B. S. (2001). Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann. N.Y. Acad. Sci. 933: 265-277.
McKay, T., Reynolds, P., Jezzard, S., Curiel, D., and Coutelle, C. (2002). Secretin-mediated gene delivery, a specific targeting mechanism with potential for treatment of biliary and pancreatic disease in cystic fibrosis. Mol. Ther. 5(4): 447-454.
Meaney, M. J., Aitken, D. H., Bhatnagar, S., and Sapolsky, R. M. (1991). Postnatal handling attenuates certain neuroendocrine, anatomical, and cognitive dysfunctions associated with aging in female rats. Neurobiol. Aging. 12(1): 31-38.
Meaney, M. J., Aitken, D. H., van Berkel, C., Bhatnagar, S., and Sapolsky, R. M. (1988). Effect of neonatal handling on age-related impairments associated with the hippocampus. Science 239(4841, Pt. 1): 766-768.
Militerni, R., Bravaccio, C., Falco, C., Fico, C., and Palermo, M. T. (2002). Repetitive behaviors in autistic disorder. Eur. Child Adolesc. Psychiatry 11(5): 210-218.
Miller, T. A., Llanos, O. L., Swierczek, J. S., Rayford, P. L., and Thompson, J. C. (1978). Concentrations of gastrin and secretin in the alimentary tract of the cat. Surgery 83(1): 90-93.
Mineo, H., Oyamada, T., and Kato, S. (1990). Effect of feeding on plasma secretin concentrations in sheep. Res. Vet. Sci. 49(2): 157-179.
Mutt, V., Carlquist, M., and Tatemoto, K. (1979). Secretin-like bioactivity in extracts of porcine brain. Life Sci. 25(20): 1703-1707.
Nelson, K. B., Grether, J. K., Croen, L. A., Dambrosia, J. M., Dickens, B. F., Jelliffe, L. L., Hansen, R. L., and Phillips, T. M. (2001). Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann. Neurol. 49(5): 597-606.
Ng, S. S., Yung, W. H., and Chow, B. K. (2002). Secretin as a neuropeptide. Mol. Neurobiol. 26(1): 97-107.
Nussdorfer, G. G., Bahcelioglu, M., Neri, G., and Malendowicz, L. K. (2000). Secretin, glucagon, gastric inhibitory polypeptide, parathyroid hormone, and related peptides in the regulation of the hypothalamus-pituitary-adrenal axis. Peptides 21(2): 309-324.
O'Donohue, T. L., Charlton, C. G., Miller, R. L., Boden, G., and Jacobowitz, D. M. (1981). Identification, characterization, and distribution of secretin immunoreactivity in rat and pig brain. Proc. Natl. Acad. Sci. U.S.A. 78(8): 5221-5224.
Oektedalen, O., Opstad, P. K., Schaffalitzky de Muckadell, O. B. (1982). Secretin—A new stress hormone? Regul. Pept. 4(4): 213-219.
Ogai, M., Matsumoto, H., Suzuki, K., Ozawa, F., Fukuda, R., Uchiyama, I., Suckling, J., Isoda, H., Mori, N., and Takei, N. (2003). fMRI study of recognition of facial expressions in high-functioning autistic patients. Neuroreport 14(4): 559-563.
Ohta, M., Funakoshi, S., Kawasaki, T., and Itoh, N. (1992). Tissue-specific expression of the rat secretin precursor gene. Biochem. Biophys. Res. Commun. 183(2): 390-395.
Owley, T., McMahon, W., Cook, E. H., Laulhere, T., South, M., Mays, L. Z., Shernoff, E. S., Lainhart, J., Modahl, C. B., Corsello, C., Ozonoff, S., Risi, S., Lord, C., Leventhal, B. L., and Filipek, P. A. (2001). Multisite, double-blind, placebo-controlled trial of porcine secretin in autism. J. Am. Acad. Child Adolesc. Psychiatry 40(11): 1293-1299.
Paquette, T. L., Shulman, D. F., Alpers, D. H., and Jaffe, B. M. (1982). Postnatal development of intestinal secretin in rats and guinea pigs. Am. J. Physiol. 243(6): G511-G5117.
Park, Y. D. (2003). The effects of vagus nerve stimulation therapy on patients with intractable seizures and either Landau-Kleffner syndrome or autism. Epilepsy Behav. 4(3): 286-290.
Paxinos, G., and Watson, C. (1998). The Rat Brain in Stereotaxic Coordinates, Academic Press, New York.
Porges, S. W. (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology 32(4): 301-318.
Posey, D. J., and McDougle, C. J. (2000). The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harv. Rev. Psychiatry 8(2): 45-63.
Richer, J. M., and Coss, R. G. (1976). Gaze aversion in autistic and normal children. Acta Psychiatr. Scand. 53(3): 193-210.
Rinaman, L., Levitt, P., and Card, J. P. (2000). Progressive postnatal assembly of limbic-autonomic circuits revealed by central transneuronal transport of pseudorabies virus. J. Neurosci. 20(7): 2731-2741.
Roberts, W., Weaver, L., Brian, J., Bryson, S., Emelianova, S., Griffiths, A. M., MacKinnon, B., Yim, C., Wolpin, J., and Robinson, T. W. (2001). Homeopathic secretin in autism: A clinical pilot study. Br. Homeopath. J. 90(2): 86-91.
Rogers, I. M., Davidson, D. C., Lawrence, J., and Buchanan, K. D. (1975). Neonatal secretion of secretin. Arch. Dis. Child 50(2): 120-122.
Ruggiero, D. A., Ross, C. A., Anwar, M., Park, D. H., Joh, T. H., and Reis, D. J. (1985). Distribution of neurons containing phenylethanolamine N-methyltransferase in medulla and hypothalamus of rat. J. Comp. Neurol. 239(2): 127-154.
Ruggiero, D. A., Welch-Horan, T. B., Keune, J. D., Anwar, N., Anwar, M., Ludwig, R. J., and Welch, M. G. (2003). Secretin: Distribution, Specificity Support Neuroregulatory Role in Autism. In 33rd Annual Meeting, Neurosci Abstracts.
Rumsey, J. M., and Ernst, M. (2000). Functional neuroimaging of autistic disorders. Ment. Retard. Dev. Disabil. Res. Rev. 6(3): 171-179.
Samson, W. K., Lumpkin, M. D., and McCann, S. M. (1984). Presence and possible site of action of secretin in the rat pituitary and hypothalamus. Life Sci. 34(2): 155-163.
Sandler, A. D., Sutton, K. A., DeWeese, J., Girardi, M. A., Sheppard, V., and Bodfish, J. W. (1999). Lack of benefit of a single dose of synthetic human secretin in the treatment of autism and pervasive developmental disorder. N. Engl. J. Med. 341(24): 1801-1806.
Schultz, R. T., Grelotti, D. J., Klin, A., Kleinman, J., Van der Gaag, C., Marois, R., and Skudlarski, P. (2003). The role of the fusiform face area in social cognition: Implications for the pathobiology of autism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358(1430): 415-427.
Steptoe, A., Cropley, M., and Joekes, K. (1999). Job strain, blood pressure and response to uncontrollable stress. J. Hypertens. 17(2): 193-200.
Strauss, E., and Yalow, R. S. (1978). Immunoreactive secretin in gastrointestinal mucosa of several mammalian species. Gastroenterology 75(3): 401-404.
Sved, A. F., Mancini, D. L., Graham, J. C., Schreihofer, A. M., and Hoffman, G. E. (1994). PNMT-containing neurons of the C1 cell group express c-fos in response to changes in baroreceptor input. Am J Physiol. 266(2, Pt. 2): R361-R367.
Swanson, L. W. (1998). Brain Maps: Structure of the Rat Brain, 2nd Revision, Elsevier Science B. V., Amsterdam, the Netherlands.
Talman, W. T., and Kelkar P. (1993). Neural control of the heart. Central and peripheral. Neurol. Clin. 11(2): 239-256.
Tanoue, Y., and Oda, S. (1989). Weaning time of children with infantile autism. J. Autism Dev. Disord. 19(3): 425-434.
Teufel, M., Luik, G., and Niessen, K. H. (1986). [Gastrin, secretin, VIP and motilin in children with mucoviscidosis and Crohn disease]. Monatsschr. Kinderheilkd. 134(3): 132-137.
Tinbergen, N., and Tinbergen, E. A. (1983). Autistic Children—New Hope for a Cure, George, Allen and Unwin, London.
Torrente, F., Ashwood, P., Day, R., Machado, N., Furlano, R. I., Anthony, A., Davies, S. E., Wakefield, A. J., Thomson, M. A., Walker-Smith, J. A., and Murch, S. H. (2002). Small intestinal enteropathy with epithelial IgG and complement deposition in children with regressive autism. Mol. Psychiatry 7(4): 375-382.
Uno, H., Tarara, R., Else, J. G., Suleman, M. A., and Sapolsky, R. M. (1989). Hippocampal damage associated with prolonged and fatal stress in primates. J. Neurosci. 9(5): 1705-1711.
Vacher, C. M., Fretier, P., Creminon, C., Calas, A., and Hardin-Pouzet, H. (2002). Activation by serotonin and noradrenaline of vasopressin and oxytocin expression in the mouse paraventricular and supraoptic nuclei. J Neurosci. 22(5): 1513-1522.
Veerendra-Kumar, M. H., and Gupta, Y. K. (2002). Intracerebroventricular administration of colchicine produces cognitive impairment associated with oxidative stress in rats. Pharmacol. Biochem. Behav. 73(3): 565-571.
Walker, J. K., Premont, R. T., Barak, L. S., Caron, M. G., and Shetzline, M. A. (1999). Properties of secretin receptor internalization differ from those of the beta(2)-adrenergic receptor. J. Biol. Chem. 274(44): 31515-31523.
Warren, R. P., Odell, J. D., Warren, W. L., Burger, R. A., Maciulis, A., Daniels, W. W., and Torres, A. R. (1997). Brief Report: Immunoglobulin A deficiency in a subset of autistic subjects. J. Autism Dev. Disord. 27(2): 187-192.
Wasserman, A. M., Ferreira, M., Jr., Sahibzada, N., Hernandez, Y. M., and Gillis, R. A. (2002). GABA-mediated neurotransmission in the ventrolateral NTS plays a role in respiratory regulation in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283(6): R1423-R1441.
Welch, M. G. (1983a). Retrieval from autism through mother-child holding. In Tinbergen, N., and Tinbergen, E. A., Autistic Children—New Hope for a Cure, George, Allen and Unwin, London.
Welch, M. G. (1983b). Retrieval from autism through mother-child holding therapy. In Call, J. D., Galenson, E., and Tyson, R. L. (eds.), Frontiers of Infant Psychiatry, Basic Books, New York, 450.
Welch, M. G. (1987). Toward prevention of developmental disorders. Pa Med. 90(3): 47-52.
Welch, M. G. (1988). Holding Time, Simon and Schuster, New York.
Welch, M. G. (1989). Holding Time: How When Why. In Proceedings of the First International Congress of Holding Therapy, Regensberg, Germany.
Welch, M. G., and Chaput P. (1988). Mother-child holding therapy and autism. Pa Med. 91(10): 33-38.
Welch, M. G., Keune, J. D., Welch-Horan, T. B., Anwar, M., Anwar, N., and Ruggiero, D. A. (2003a). Secretin activates visceral brain regions in rat including areas abnormal in autism. Cell Mol Neurobiol. 23 (4/5): 817-837.
Welch, M. G., Welch-Horan, T. B., Keune, J. D., Anwar, N., Anwar, M., Ludwig, R. J., and Ruggiero, D. A. (2003b). Neurohormonal resolution of genetic and acquired IBD and secondary brain activation in areas abnormal in autism. In 33rd Annual Meeting, Neuroscience Abstracts.
Welch, M. G., Keune, J. D., Welch-Horan, T. B., and Ruggiero, D. A. (2002a). Secretin in autism. Soci. Neurosci. Press Book, 32nd Annu. Meet. II: 572-574.
Welch, M. G., Keune, J. D., Welch-Horan, T. B., and Ruggiero, D. A. (2002b). Secretin activates visceral brain regions including areas abnormal in autism. In 32nd Annual Meeting, Neuroscience Abstracts.
Wheeler, G. (2003). RG-1068 RepliGen. Curr. Opin. Investig. Drugs 4(1): 66-71.
White, J. F. (2003). Intestinal pathophysiology in autism. Exp. Biol. Med. (Maywood) 228(6): 639-649.
Yung, W. H., Leung, P. S., Ng, S. S., Zhang, J., Chan, S. C., and Chow, B. K. (2001). Secretin facilitates GABA transmission in the cerebellum. J. Neurosci. 21(18): 7063-7068.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Welch, M.G., Keune, J.D., Welch-Horan, T.B. et al. Secretin: Hypothalamic Distribution and Hypothesized Neuroregulatory Role in Autism. Cell Mol Neurobiol 24, 219–241 (2004). https://doi.org/10.1023/B:CEMN.0000018618.59015.a2
Issue Date:
DOI: https://doi.org/10.1023/B:CEMN.0000018618.59015.a2