Abstract
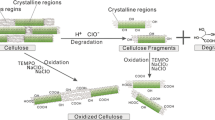
The kinetics of the TEMPO-mediated oxidation of regenerated celluloses has been studied. It is revealed that the oxidation reaction of the regenerated celluloses by the 2,2,6,6-tetramethyl-piperidine-1-oxyl radical (TEMPO)–NaBr–NaOCl system can be approximately described by first-order kinetics with respect to substrate. In the concentration range used the rate constant k is directly proportional to the concentration of TEMPO, while it is proportional to the concentration of NaBr in a relatively lower range and tends to level off at higher concentration. The effect of temperature on the rate constant can be well described by the Arrhenius equation, the apparent activation energy measured is about 66.2 kJ/mol. The effect of the pH of the reaction solution, the crystallinity and morphology of the substrates on the oxidation rate is also discussed.
Similar content being viewed by others
References
Chang P.S. and Robyt J.F. 1996. Oxidation of primary alcohol groups of naturally occurring polysaccharides with 2,2,6,6-tetramethyl-1-piperidine oxoammoniumion. J. Carbohydr. Chem. 15: 819-830.
Chang P.S. and Robyt J.F. 1998. Oxidation of the primary alcohol groups of cyclomaltodextrins with 2,2,6,6-tetramethyl-1-piperidine oxoammoniumion. Carbohydr. Lett. 3: 31-38.
De Nooy A.E., Besemer A.C. and van Bekkum H. 1994. Highly selective TEMPO-mediated oxidation of primary alcohol groups in polysaccharides. Recl. Trav. Chim. Pays-Bas 113: 165-166.
De Nooy A.E., Besemer A.C. and van Bekkum H. 1995a. Highly selective nitroxyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans. Carbohydr. Res. 269: 89-98.
De Nooy A.E., Besemer A.C. and van Bekkum H. 1995b. Selective oxidation of primary alcohols mediated by nitroxyl radical in aqueous solution. Kinetics and mechanism. Tetrahedron 51: 8023-8032.
De Nooy A.E., Besemer A.C., van Bekkum H., van Dijk J.A.P.P. and Smit J.A.M. 1996. TEMPO-mediated oxidation of pullulan and in influence of ionic strength and linear charge density on the dimensions of the obtained polyelectrolyte chains. Macromolecules 29: 6541-6547.
Farkas L., Lewin M. and Bloch R. 1949. The reaction between hypochlorite and bromides. J. Am. Chem. Soc. 71: 1988-1991.
Friedlander B.I., Dutt A.S. and Rapson W.H. 1966. The infrared spectra of oxidized cellulose, part III, sodium hypochlorite oxidation. Tappi 49(10): 468-472.
Ibert M., Marsais F., Merbouh N. et al. 2002. Determination of the side-products formed during the nitro-mediated bleach oxidation of glucose to glucaric acid. Carbohydr. Res. 337: 1059-1063.
Isogai A. and Kato Y. 1998. Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 5: 153-164.
Lenz J., Schurz J. and Wrentachur E. 1993. Properties and structure of solvent-spun and viscose-type bers in the swollen state. Colloid Polym. Sci. 271(5): 460-468.
Kato Y., Matsuo R. and Isogai A. 2003. Oxidation process of water-soluble starch in TEMPO-mediated system. Carbohydr. Polym. 51: 69-75.
Kumar K. and Margerum D.W. 1987. Kinetics and mechanism of general-acid-assisted oxidation of bromide by hypochlorite and hypochlorous acid. Inorg. Chem. 26: 2706-2711.
Nevell T.P. 1951. Oxidation of cotton cellulose by nitrogen dioxide. J. Textile Inst. 42: T91-T129.
Painter T.J. 1977. Preparation and periodate oxidation of C-6-oxycellulose:conformational interpretation of hemiacetal stability. Carbohydr. Res. 55: 95-103.
Painter T.J. 1985. New glucuronoglucans obtained by oxidation of amylose at position 6. Carbohydr. Res. 140: 61-68.
Rahn K. and Heinze T. 1998. New cellulosic polymers by subsequent modication of 2,3-dialdehyde cellulose. Cell. Chem. Technol. 32: 173-183.
Shibata I. and Isogai A. 2003. Depolymerization of celluronic acid during TEMPO-mediated oxidation. Cellulose 10: 151-158.
Tahiri C. and Vignon M.R. 2000. TEMPO-oxidation of cellulose:synthesis and characterization of polyglucuronans. Cellulose 7: 177-188.
Unruh C.C. and Kenyon W.O. 1942. Investigation of the properties of cellulose oxidized by nitrogen dioxide. J. Am. Chem. Soc. 64: 127-131.
Varma A.J. and Chavan V.B. 1995. Some preliminary studies on polyelectrolyte and rheological properties of sodium 2,3-dicarboxy-cellulose. Carbohydr. Polym. 27: 63-67.
Yackel E.C. and Kenyon W.O. 1942. Oxidation of cellulose by nitrogen dioxide. J. Am. Chem. Soc. 64: 121-127.
Yalpani M. 1985. A survey of recent advances in selective chemical and enzymic polysaccharide modications. Tetrahedron 41: 2957-3020.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sun, B., Gu, C., Ma, J. et al. Kinetic study on TEMPO-mediated selective oxidation of regenerated cellulose. Cellulose 12, 59–66 (2005). https://doi.org/10.1023/B:CELL.0000049409.56806.da
Issue Date:
DOI: https://doi.org/10.1023/B:CELL.0000049409.56806.da