Abstract
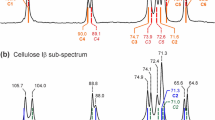
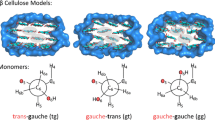
The chemical shift anisotropies (CSAs) of cellulose Iα and Iβ, the two crystalline constituents of bacterial cellulose produced by Acetobacter xylinum (DSM 14666), and regenerated cellulose II are reported for each of the spectroscopically resolved carbon resonances using the phase adjusted spinning sideband (PASS) experiment. The data are compared with experimental results using the recoupling of anisotropy information (RAI) technique and with theoretical calculations of the structure of cellulose, including the hydrogen bonding systems.
Similar content being viewed by others
References
Alderman D.W., McGeorge G., Hu J.Z., Pugmire R.J. and Grant D.M. 1998. A sensitive, high resolution magic angle turning experiment for measuring chemical shift tensor principal values. Mol. Phys. 95(6): 1113-1126.
Antzutkin O.N., Shekar S.C. and Levitt M.H. 1995. Two-dimensional sideband separation in magic-angle-spinning NMR. J. Magn. Reson. A 115: 7-19.
Atalla R.H. and Gast J.C. 1980. 13C NMR spectra of cellulose polymorphs. J. Am. Chem. Soc. 102(9): 3249-3251.
Atalla R.H. and VanderHart D.L. 1984. Native cellulose: a composite of two distinct crystalline forms. Science 223: 283-284.
Bax A., Szeverenyi N.M. and Maciel G.E. 1983a. Correlation of isotropic shifts and chemical shift anisotropies by twodimensional Fourier-transform magic-angle hopping NMR spectroscopy. J. Magn. Reson. 52: 147-152.
Bax A., Szeverenyi N.M. and Maciel G.E. 1983b. Chemical shift anisotropy in powdered solids studied by 2D FT NMR with flipping of the spinning axis. J. Magn. Reson. 55: 494-497.
Dudley R.L., Fyfe C.A., Stephenson P.J., Deslandes Y., Hamer G.K. and Marchessault R.H. 1983. High-resolution 13C CP/ MAS NMR spectra of solid cellulose oligomers and the structure of cellulose II. J. Am. Chem. Soc. 105(8): 2469-2472.
Earl W.L. and VanderHart D.L. 1980. High resolution magic angle sample spinning 13C NMR of solid cellulose I. J. Am. Chem. Soc. 102: 3251-3252.
Earl W.L. and VanderHart D.L. 1982. Measurement of 13C chemical shifts in solids. J. Magn. Reson. 48: 35-54.
Erata T., Shikano T., Yunoki S. and Takai M. 1997. The complete assignment of the 13C CP/MAS NMR spectrum of native cellulose by using 13C labeled glucose. Cellulose Commun. 4: 128-131.
Gagnaire D., Mancier D. and Vincendon M. 1980. Cellulose organic solutions: a nuclear magnetic resonance investigation. J. Polym. Sci. Polym. Chem. Ed. 18(1): 13-25.
Gan Z. 1994. Spinning-sideband suppression using a pseudotwo-dimensional experiment. J. Magn. Reson. 109: 253-255.
Gast J.C., Atalla R.H. and McKelvey R.D. 1980. The Carbon-13 NMR spectra of the xylo-and cellooligosaccharides. Carbohydr. Res. 84(1): 137-146.
Herzfeld J. and Berger A.E. 1980. Sideband intensities in NMR spectra of samples spinning at the magic angle. J. Chem. Phys. 73(12): 6021-6030.
Hesse St. 1998. NMR-Untersuchungen von Cellulosen und Biopolymeren. Diploma Thesis, IOQ/HF, FSU Jena, Germany.
Horii F., Hirai A. and Kitamaru R. 1983. Solid-state 13C-NMR study of conformations of oligosaccharides and cellulose: conformation of CH2OH group about the exo-cyclic C–C bond. Polym. Bull. 10: 357-361.
Hu J.Z., Wang W., Liu F., Solum M.S., Alderman D.W., Pugmire R.J. and Grant D.M. 1995. Magic-angle-turning experiments for measuring chemical-shift-tensor principal values in powdered solids. J. Magn. Reson. A 133: 210-222.
Inoue Y. and Chujo R. 1978. The carbon-13 NMR spectra of (1 → 4)-linked β-D-gluco-oligosaccharides. Carbohydr. Res. 60(2): 367-370.
Jaeger C., Pauli J. and Schmauder H.-P. 2004. Observation of the glycosidic bond and complete ring assignment of the carbons in uniformly 13C labeled bacterial cellulose. Macromolecules, submitted.
Koch F.-Th., Priess W., Witter R. and Sternberg U. 2000. Calculation of solid-state 13C NMR spectra of cellulose Iα, Iβ and II using a semi-empirical approach and molecular dynamics. Macromol. Chem. Phys. 201: 1930-1939.
Kolpak F.J. and Blackwell J. 1976. Determination of the structure of cellulose II. Macromolecules 9: 273-278.
Kono H., Yunoki S., Shikano T., Fujiwara M., Erata T. and Takai M. 2002. CP/MAS 13C NMR study of cellulose and cellulose derivatives. 1. Complete assignment of the CP/MAS 13C NMR spectrum of the native cellulose. J. Am. Chem. Soc. 124: 7506-7511.
Kono H., Erata T. and Takai M. 2003. Determination of the through-bond carbon–carbon and carbon–proton connectivities of the native celluloses in the solid state. Macromolecules 36: 5131-5138.
Langan P., Nishiyama Y. and Chanzy H. 1999. A revised structure and hydrogen-bonding system in cellulose II from a neutron fiber diffraction analysis. J. Am. Chem. Soc. 121: 9940-9946.
Lesage A., Bardet M. and Emsley L. 1999. Through-bond carbon–carbon connectivities in disordered solids by NMR. J. Am. Chem. Soc. 121: 10987-10993.
Maciel G.E., Szeverenyi N.M. and Sardashti M. 1985. Chemical-shift-anisotropy powder patterns by the two-dimensional angle-flipping approach. Effects of crystallite packing. J. Magn. Reson. 64: 365-374.
Mason J. 1993. Convention for the reporting of nuclear magnetic shielding (or shifts) tensors suggested by participants in the NATO ARW on NMR shielding constants at the University of Maryland, College Park, July 1992. Solid State Nucl. Magn. Res. 2: 285-288.
Nishiyama Y., Langan P. and Chanzy H.J. 2002. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 124: 9074-9082.
Nishiyama Y., Sugiyama J., Chanzy H. and Langan P. 2003. Crystal structure and hydrogen bonding system in cellulose Iα from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 125: 14300-14306.
Raymond S., Kvick A. and Chanzy H. 1995. The structure of cellulose II: a revisit. Macromolecules 28: 8422-8425.
Schramm M. and Hestrin S. 1954. Factors a.ecting production of cellulose at the air/liquid interface of a culture of Acetobacter xylinum. J. Gen. Microbiol. 11: 123-129.
Sternberg U. and Möllho. M. 2001. Molecular mechanics with fluctuating atomic charges–a new force field with a semi-empirical charge calculation. J. Mol. Model. 7: 90-102.
Sternberg U., Koch F.-Th., Priess W. and Witter R. 2003. Crystal structure refinements of cellulose polymorphs using solid-state 13C chemical shifts. Cellulose 10: 189-199.
Sugiyama J., Persson J. and Chanzy H. 1991a. Combined infrared and electron diffraction study of the polymorphism of native celluloses. Macromolecules 24: 2461-2466.
Sugiyama J., Vuong R. and Chanzy H. 1991b. Electron diffraction study on the two crystalline phases occurring in native cellulose from an algal cell wall. Macromolecules 24: 4168-4175.
Szeverenyi N.M., Bax A. and Maciel G.E. 1985. Magic-angle hopping as an alternative to magic-angle spinning for solid state NMR. J. Magn. Reson. 61: 440-447.
Tycko R., Dabbagh G. and Mirau P.A. 1989. Determination of chemical-shift-anisotropy lineshapes in a two-dimensional magic-angle-spinning NMR experiment. J. Magn. Reson. 85: 265-274.
VanderHart D.L. and Atalla R.H. 1984. Studies of microstructure in native cellulose using solid-state 13C NMR. Macromolecules 17: 1465-1472.
VanderHart D.L. and Atalla R.H. 1987. Further carbon-13 NMR evidence for the coexistence of two crystalline forms in native celluloses. In: Atalla R.H. (ed.), The Structures of Celluloses. ACS Symp. Ser., 340. American Chemical Society, Washington, DC, pp. 88-118.
Wickholm K., Hult E.-L., Larsson P.T., Iversen T. and Lennholm H. 2001. Quantification of cellulose forms in complex cellulose materials: a chemometric model. Cellulose 8: 139-148.
Witter R., Hesse St. and Sternberg U. 2003. New powder pattern recoupling at 10 kHz spinning speed applied to cellulose. J. Magn. Reson. 161: 35-42.
Zollfrank C. 1999. Darstellung von Cellulose-II-Einkristallen und Untersuchung ihrer Kristallstruktur. Dissertation. HFM, TU München, Germany.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hesse, S., Jäger, C. Determination of the 13C chemical shift anisotropies of cellulose I and cellulose II. Cellulose 12, 5–14 (2005). https://doi.org/10.1023/B:CELL.0000049407.56737.c7
Issue Date:
DOI: https://doi.org/10.1023/B:CELL.0000049407.56737.c7