Abstract
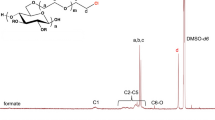
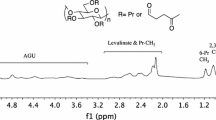
Functional thiomethyl and thiosulfate derivatives of carboxymethylcellulose (CMC, DS = 0.9) were synthesized by nucleophilic displacement reactions. Alkylation of CMC by allyl glycidyl ether took mainly place at the primary positions of the cellulose backbone and yielded a 6-O-(3′-allyloxy-2′-hydroxypropyl)-CMC 1 with a partial DS of 3′-allyloxy-2′-hydroxypropyl substituents DSallyl of up to 0.43. Addition of tetrathionate to the allyl groups gave rise to 6-O-(2′′,3′′-bis(thiosulfato)propoxy-2′-hydroxypropyl)-CMC 2. As the addition of tetrathionate was sluggish and incomplete, alternatively bromine was added and the resulting dibromide was substituted by thiosulfate. A 40% conversion of the allyl groups was achieved by this two-step procedure. On the other hand, the addition of bromine to 1 in aqueous solution almost quantitatively yielded the bromohydrin derivative which was converted by displacement reaction with thiosulfate to 6-O-(2′′-hydroxy-3′′-thiosulfatopropoxy-2′-hydroxypropyl)-CMC 4. 6-Thiomethyl-6-deoxy-CMC 6 was synthesized by displacement reaction of 6-O-tosylcellulose with sodium methylsulfide and subsequent carboxymethylation of the cellulose backbone. A partial DS of thiomethyl substituents DSThM=0.65 exclusively at the primary positions was obtained. All functional CMC derivatives, 2, 4, and 6 were readily available in gram quantities, rather stable and highly water soluble for pH > 3. On gold surfaces they form self-assembled monolayers (SAMs) with thicknesses of 1.2 to 2.4 nm as determined by surface plasmon resonance (SPR).
Similar content being viewed by others
References
Axen R.E.A.V., Porath J.O. and Carlsson P.J.E. 1975. Thiopolymers and derivatives. Patent DE 2523793, Exploaterings AB T.B.F. Chem. Abstr. 84, 151744.
Baker A.A., Helbert W., Sugiyama J. and Miles M.J. 2000. New insight into cellulose structure by atomic force microscopy shows the Iα crystal phase at near-atomic resolution. Biophys. J. 79: 1139-1145.
Beulen M.W.J., Huisman B.-H., van der Heijden P.A., van Veggel F.C.J.M., Simons M.G., Biemond E.M.E.F., de Lange P.J. and Reinhoudt D.N. 1996. Evidence for nondestructive adsorption of dialkyl sul des on gold. Langmuir 12: 6170-6172.
Choi S., Lauer H., Wenz G., Bruns M. and Petri D.F.S. 2000. Formation of dense cellulose monolayers on silver surfaces. J. Braz. Chem. Soc. 11: 11-15.
Distler H. 1967. The chemistry of bunte salts. Angew. Chem., Int. Ed. Engl. 6: 544-553.
Heuser E., Heath M. and Shochley W.H. 1950. The rate of esterication of primary and secondary hydroxyls of cellulose with p-toluenesulfonyl (tosyl)chloride. J. Am. Chem. Soc. 72: 670-674.
Ho F.F.L. and Klosiewicz D.W. 1980. Proton nmr spectrometry for determination of substituents and their distribution in carboxymethylcellulose. Anal.Chem. 52: 913-916.
Itoh T., Tsujii Y., Suzuki H., Fukuda T. and Miyamoto T. 1992. Mono-and multilayer Langmuir-Blodgett films of cellulose tri-n-alkyl esters study by transmission electron microscopy. Polym. J. 24: 641-652.
Jaehne E., Kowalik T., Adler H.-J., Plagge A. and Stratmann M. 2002. Ultra-thin layers of phosphorylated cellulose derivatives on metal surfaces. Macromol. Symp. 177: 97-109.
Kamide K. and Saito M. 1983. Persistence length of cellulose and cellulose derivatives in solution. Macromol. Chem. Rapid Commun. 4: 33-39.
Kawaguchi T., Nakahara H. and Fukuda K. 1985. Mono-and multilayers of amphiphilic cellulose esters and some novel comblike polymers. J. Colloid Interface Sci. 104: 290-293.
Khan A.R., Barton L. and T. D'Souza V. 1996. Epoxides of the secondary side of cyclodextrins. J. Am. Chem. Soc. 61: 8301-8303.
Klemm D., Heinze T., Philipp B. and Wagenknecht W. 1997. New approaches to advanced polymers by selective cellulose functionalization. Acta Polym. 48: 277-297.
Kowalik T., Adler H.-J., Plagge A. and Stratmann M. 2000. Ultrathin layers of phosphorylated cellulose derivatives on aluminium surfaces. Macromol. Chem. Phys. 15: 2064-2069.
Kulicke W.-M., Kull A.H., Kull W., Thielking H., Engelhardt J. and Pannek J.-B. 1996. Characterization of aqueous carboxymethylcellulose solutions in terms of their molecular structure and its influence on rheological behaviour. Polymer 37: 2723-2731.
Lehr B., Seufert M., Wenz G. and Decher G. 1996. Fabrication of poly(p-phenylene vinylene) (ppv) nano-heterocomposite films via layer-by-layer adsorption. Supramol. Sci. 2: 199-207.
Lynch D.W. and Hunter W.R. 1985. Optical constants of metals. In: Palik E.D. (ed.), Handbook of Optical Constants of Solids. Academic Press, San Diego, CA, pp. 286-295.
Mays J.W. 1988. Solution properties and chain stiffness of (cyanoethyl) (hydroxypropyl)cellulose. Macromolecules 21: 3179-3183.
Michielsen S. 1999. Specific refractive index of polymers in dilute solution/c. Refractometric calibration data. Brandrup J., Immergut E.H. and Grulke E.A., Polymer Handbook. J. Wiley & Sons, New York, pp. VII/500.
Rahn K., Diamantoglou M., Klemm D., Berghmans H. and Heinze T. 1996. Homogeneous synthesis of cellulose p-to-luenesulfonates in N,N-dimethylacetamide/LiCl solvent system. Angew. Makromol. Chem. 238: 143-163.
Schaub M., Mathauer K., Schwiegk S., Albouy P.A., Wenz G. and Wegner G. 1992. Investigation of molecular superstructures of hairy rodlike polymers by X-ray reflection. Thin Solid Films 210: 397-400.
Schaub M., Wenz G., Wegner G., Stein A. and Klemm D. 1993. Ultrathin fllms of cellulose on silicon wafers. Adv. Mater. 12: 919-921.
Takahashi S.I., Fujimoto T., Barua B.M. and Miyamoto T. 1986. 13-C-nmr spectral studies on the distribution of substituents in some cellulose derivatives. J. Polym. Sci., Part A: Polym. Chem. Ed. 24: 2981-2993.
Wenz G., Choi S., Bruns M., Schimmel T., Beyer H. and Siqueira Petri D.F. 1999. Synthesis of a cellulose thiosulfate and its immobilization on gold surfaces. Polymer 40: 1593-1601.
Wenz G., Petri D.F. and Choi S.W. 2001. Polymer thiosulfates for coating of metals. Patent US 6,245,579 B1 Universität Karlsruhe, Chem.Abstr. 130, 183895.
Zynio S.A., Samoylov A.V., Surovtseva E.R., Mirsky V.M. and Shirshov Y.M. 2002. Bimetallic layers increase sensitivity of affinity sensors based on surface plasmon resonance. Sensors 2: 62-70.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wenz, G., Liepold, P. & Bordeanu, N. Synthesis and SAM formation of water soluble functional carboxymethylcelluloses: thiosulfates and thioethers. Cellulose 12, 85–96 (2005). https://doi.org/10.1023/B:CELL.0000049406.09515.83
Issue Date:
DOI: https://doi.org/10.1023/B:CELL.0000049406.09515.83