Abstract
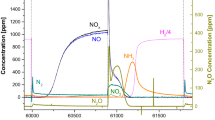
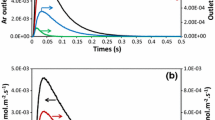
For elucidating the mechanistic aspects of oxygen formation during N2O decomposition over commercial woven Pt–Rh gauze, transient experiments were carried out in the temporal analysis of products (TAP) reactor by pulsing N2 16O over 18O-pretreated gauze catalyst at temperatures typical of industrial ammonia burners (1073–1273 K). The transient responses of N2O and the products of its decomposition (O2 and N2) were fitted to two different mechanistic models. From the isotopic studies and the fitting of transient experiments, two separate routes of oxygen formation during catalytic N2O decomposition have been identified. Oxygen is produced via both (i) interaction of N2O with adsorbed oxygen species formed from N2O and (ii) recombination of adsorbed oxygen species on the catalyst surface. The relative contribution of these reaction pathways depends on the reaction temperature.
Similar content being viewed by others
References
J. Perez-Ramirez, F. Kapteijn, G. Mul, X. Xu and J. A. Moulijn, Catal. Today 76 (2002) 55.
J. Perez-Ramírez, F. Kapteijn, K. Schoffel and J. A. Moulijn, Appl. Catal. B: Environ. 44 (2003) 117.
C. N. Hinshelwood and C. R. Prichard, J. Chem. Soc. 127 (1925) 327.
L. Riekert and M. Staib, Berichte der Bunsengesellschaft 67 (1963) 976.
C. G. Takoudis and L. D. Schmidt, J. Catal. 80 (1983) 274.
E. W. R. Steacie and J. W. McCubbin, J. Chem. Phys. 2 (1934) 585.
E. W. R. Steacie and J. W. McCubbin, Can. J. Res. 14B (1936) 84.
T. Hahn and H.-G. Lintz, Zeitschrift fur Physikalische Chemie 186 (1994) 195.
L. Riekert, D. Menzel and M. Staib, 3rd International Congress on Catalysis, 1965, p. 387.
S.-I. Tanaka, K. Yuzaki, S.-I. Ito, H. Uetsuka, S. Kameoka and K. Kunimori, Catal. Today 63 (2000) 413.
S.-I. Tanaka, K. Yuzaki, S.-I. Ito, S. Kameoka and K. Kunimori, J. Catal. 200 (2001) 203.
F. Kapteijn, J. Rodriguez-Mirasol and J. A. Moulijn, Appl. Catal. B: Environ. 9 (1996) 25.
J. T. Gleaves, G. S. Yablonsky, P. Phanawadee and Y. Schuurman, Appl. Catal. A: Gen. 160 (1997) 55.
M. Soick, D. Wolf and M. Baerns, Chem. Eng. Sci. 55 (2000) 2875.
E. V. Kondratenko, R. Krahnert, M. Baerns and J. Perez-Ramirez (in preparation).
G. A. Papapolymerou and L. D. Schmidt, Langmuir 1 (1985) 488.
C. T. Campbell, G. Ertl, H. Kuipers and J. Segner, Surf. Sci. 107 (1981) 220.
D. H. Parker, M. E. Bartram and B. E. Koel, Surf. Sci. 217 (1989) 489.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kondratenko, E.V., Pérez-Ramírez, J. Transient Studies of Direct N2O Decomposition over Pt–Rh Gauze Catalyst. Mechanistic and Kinetic Aspects of Oxygen Formation. Catalysis Letters 91, 211–216 (2003). https://doi.org/10.1023/B:CATL.0000007157.87417.fe
Issue Date:
DOI: https://doi.org/10.1023/B:CATL.0000007157.87417.fe