Abstract
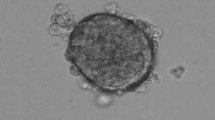
Once human islets are isolated, they are typically transplanted into type 1 diabetic recipients within 2 h of isolation. This time restriction makes it difficult for patients to travel from distant locations to receive an islet transplant and it also makes it difficult to complete pre-release quality control assessments (i.e., endotoxin and gram stain) before the expiration of the islet product. Therefore, there were two goals for this study. The first was to measure the stability of islets after a 24 h culture period using CMRL media 1066 (CMRL) supplemented with either fetal bovine serum (FBS); albumin or insulin transferrin and selenium (ITS). The second was to determine the impact of cell concentration and media depth on islet stability. The results of the study indicated that culture recoveries at 37 °C with CMRL + ITS (also known as Memphis media) were higher (64.1 ± 8.3%) than with CMRL supplemented with FBS (38.7 ± 9.7%) or albumin (47.6 ± 8.2%) and that post-culture islet viabilities, post-culture purities and stimulation indexes (SIs) were comparable. In the second series of experiments, the results showed that islets recoveries and SIs in cultures with low islet concentrations (300 IE/ml) were significantly better than cultures at high islet concentrations (1500 IE/ml). Additionally, at a shallow media depth (1.4 vs. 7 mm of media) the SI of the islets improved, and this effect was independent of the additive (i.e., FBS, albumin and ITS).
Similar content being viewed by others
References
Alejandro R., Ferreira J.V., Caulfield A., Froud T., Baidal D., Geiger M., Rothenberg L., Al-Abdullah I.H., Kirlen T., Kenyon N.S. and Ricordi C. 2002. Insulin independence in 7 patients following transplantation of cultured human islets. American Journal of Transplantation 2(Suppl. 3): abstract 358.
Anderson A. 1978. Isolated mouse pancreatic islets in culture: Effects of serum and different culture media on the insulin production of the islets. Diabetologia 14: 397-404.
Bank H. 1988. Rapid assessment of islet viability with acridine orange and propidium iodide. In Vitro Cell Developmental Biology 24: 266-273.
Barnes D. and Sato G. 1980. Methods for growth of cultured cells in serum-free media. Anat. Biochem. 102: 255-270.
Brendel M.D., Hering B.J., Schultz A.O. and Bretzel R.G. 2001. International islet transplant registry newsletter #9
Di Carlo A., Scharp D.W., Gingerich R.L., Giannarelli R., Ansara M., Olack B.J., Swanson C.J. and Navalesi R. 1994. Insulin and glucagon release from isolated, perifused human islets following low-temperature culture and cryopreservation. Transplantation Proceedings 26: 821-822.
Dionne K., Colton C. and Yarmish M. 1993. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42: 12-21.
Fraga D., Sabek O., Hathaway D. and Gaber A.O. 1998. A comparison of media supplement method for the extended culture of human islet tissue. Transplantation 65: 1060-1066.
Gaber A.O., Fraga D.W., Callicutt C.S., Gerling I.C., Sabek O.M. and Kotb M.Y. 2001. Improved in vivo pancreatic islet function after prolonged in vitro islet culture. Transplantation 72: 1730-1736.
Holmes M.A., Clayton H.A., Chadwick D.R., Bell P.R., London N.J. and James R.F. 1995. Functional studies of rat, porcine, and human pancreatic islets cultured in ten commercially available media. Transplantation 60: 854-880.
Lacy P.E., Davine J.M. and Finke E.H. 1979. Prolongation of islet allograft survival following in vitro culture (24 °C) and single injection of ALS. Science 2204: 312-313.
Lacy P. and Kostianovsky M. 1976. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16: 35-39.
Lakey J.R., Warnock G.L., Kneteman N.M., Ao A. and Rajotte R.V. 1994. Effects of pre-cryopreservation culture on human islet recovery and in vitro function. Transplantation Proceedings 26: 820.
Lakey J.R., Warnock G.L., Shapiro A.M., Korbutt G.S., Ao Z., Kneteman N.M. and Rajotte R.V. 1999. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplantation 8: 285-292.
Matsumoto S., Qualley S.A., Goel S., Hagman D.K., Sweet I.R., Poitout V., Strong D.M., Robertson R.P. and Reems J.A. 2002. Effect of the two-layer (University of Wisconsin solution-Perfluorochemical plus O2) method of pancreas preservation on human islet isolation as assessed by Edmonton isolation protocol. Transplantation 74: 1414-1419.
Petkov P., Hahn H.J., Galabova R. and Ziegler M. 1974. Investigations on islets of Langerhans in vitro. Ultrastructure and insulin secretion of isolated rat islets after different digestion with collagenase. Acta Histochemistry (Jena) 51: 50-60.
Ricordi C., Lacy P.E., Sterbenz K. and Davie J.M. 1987. Lowtemperature culture of human islets or in vivo treatment with L3T4 antibody produces a marked prolongation of islet human-to-mouse xenograft survival. Proceedings of the National Academic Sciences USA 84: 8080-8084.
Ricordi C., Gray D.W., Hering B.J., Kaufman D.B., Warnock G.L., Kneteman N.M., Lake S.P., London N.J., Socci C. and Alejandro R., 1990. Islet isolation assessment in man and large animals. Acta Diabetologica Latina 27: 185-195.
Ricordi C., Lacy P.E., Finke E.H., Olack B.J. and Sharp D.W. 1988. Automated method for isolation of human pancreatic islets. Diabetes 37: 413-420.
Ryan E.A., Lakey J.R.T., Paty B.W., Imes S., Korbutt G.S., Kneteman N.M., Bigam D., Rajotte R.V. and Shapiro A.M. 2002. Successful islet transplantation. Continued insulin reserve provides long-term glycemic control. Diabetes 51: 2148-2157.
Ryan E.A., Lakey J.R., Rajotte R.V., Korbutt G.S., Kin T., Imes S., Rabinovitch A., Elliott J.F., Bigam D., Kneteman N.M., Warnock G.L. and Larsen I. 2001. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 50: 710.
Schrezenmeir J., Gero L., Solhdju M., Kirchengessner J., Laue C., Beyer J., Stier H. and Muller-Klieser W. 1994. Relation between secretory function and oxygen supply in isolated islet organs. Transplantation Proceedings 26: 809-813.
Shapiro A.M., Lakey J.R., Ryan E.A., Korbutt G.S., Toth E., Warnock G.L., Kneteman N.M. and Rajotte R.V. 2000. Islet transplantation in seven patients with Type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. New England Journal of Medicine 343: 230-238.
Sharp D.W., Lacy P.E., Finke E. and Olack B. 1987. Lowtemperature culture of human islets isolated by the distention method and purified with Ficoll or Percoll gradients. Surgery 102: 869-879.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, S., Goel, S., Qualley, S. et al. A comparative evaluation of culture conditions for short-term maintenance (<24 hr) of human islets isolated using the Edmonton protocol. Cell Tissue Banking 4, 85–93 (2003). https://doi.org/10.1023/B:CATB.0000007043.15164.8a
Issue Date:
DOI: https://doi.org/10.1023/B:CATB.0000007043.15164.8a