Abstract
Objective: The aim was to study the pharmacodynamic interactions and safety of the co-administration of the calcium sensitizer levosimendan and the calcium antagonist felodipine in patients with coronary heart disease (CHD) and with normal ejection fraction (EF).
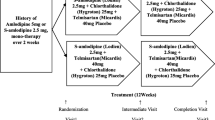
Methods: The study was a randomized, double blind, placebo-controlled, crossover study in 24 male patients with Canadian Cardiovascular Society (CCS) class II CHD, consisting of four treatment periods, each period lasting for 7–10 days. In the first period the patients received either oral levosimendan (LS) (0.5 mg) or placebo (PL) four times daily and were then crossed over to the other therapy for the second and third period. After the third period the patients were changed back to the therapy administered in the first period. Open label felodipine (FD), 5 mg once daily, was co-administered on the third and fourth treatment period. Differences between the four treatments (LS, PL, FD and LS + FD) in systolic time intervals, exercise capacity, heart rate, blood pressure and 24-hour continuous electrocardiography (Holter) were assessed.
Results: The differences between treatments regarding heart rate corrected electromechanical systole (QS2i), pre-ejection period (PEP) and heart rate corrected left ventricular ejection time (LVETi) were significant (p < 0.001, p < 0.001 and p = 0.004, respectively). Levosimendan shortened QS2i by 10 ms (95% CI [−15, −4]), PEP by 6 ms (95% CI [−10, −3]) and LVETi by 7 ms (95% CI [−13, −2]) compared with placebo, indicating a moderate positive inotropic effect. The results were similar, when levosimendan was administered concomitantly with felodipine. Levosimendan did not significantly change systolic blood pressure and no potentation of response was seen with concomitant administration with felodipine. The increase in heart rate after levosimendan and its combination with felodipine was equal (6–7 bpm). There was no difference in mean cumulative exercise time between the treatments. The combination of levosimendan and felodipine was well tolerated.
Conclusion: No clinically significant pharmacodynamic interactions between levosimendan and felodipine were seen. Levosimendan did not aggravate myocardial ischemia. Levosimendan can safely be administered to patients with CHD together with a dihydropyridine calcium antagonist.
Similar content being viewed by others
References
Pollesello P, Ovaska M, Kaivola J. Binding of a new Ca2+ sensitizer, levosimendan, to recombinant human troponin C. J Biol Chem 1994; 269(46): 28584-28590.
Haikala H, Kaivola J, Nissinen E, Wall P, Levijoki J, Linden IB. Cardiac troponin C as a target protein for a novel calcium sensitizing drug, levosimendan. J Mol Cell Cardiol 1995; 27: 1859-1866.
Lancaster MK, Cook SJ. The effects of levosimendan on [Ca2+]i in guinea-pig isolated ventricular myocytes. Eur J Pharmacol1997; 339: 97-100.
Edes I, Kiss E, Kitada Y, et al. Effects of levosimendan, a cardiotonic agent targeted to troponin C, on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in guinea pig heart. Circ Res1995; 77: 107-113.
Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. Levosimendan, a novel Ca2+-sensitizer activates the glibenclamide-sensitiveK+ channel in rat arterial myocytes. Eur J Pharmacol1997; 333: 249-259.
Hasenfuss G, Pieske B, Castell M, Kretchmann B, Maier LS, Just H. Influence of the novel inotropic agent levosimendan on isometric tension and calcium cycling in failing human myocardium. Circulation1998; 98: 2141-2147.
Kersten JR, Montgomery MW, Pagel PS, Warltier DC. Levosimendan, a new positive inotropic drug, decreases myocardial infarct size via activation of K (ATP) channels. Anesth Analg2000; 90: 5-11.
Levijoki J, Pollesello P, Kaheinen P, Haikala H. Improved survival with simendan after experimental myocardial infarction in rats. Eur J Pharmacol2001; 419: 243-248.
Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA. Mitrovic V on behalf of the Study Group. Haemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol2000; 36: 1903-1912.
Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E. Hare J on behalf of the Study Investigators. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation 2000; 102: 2222-2227.
Follath F, Cleland JGF, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): A randomised double-blind trial. Lancet2002; 360: 196-202.
Lilleberg J, Nieminen MS, Akkila J, et al. Effects of anewcalcium sensitizer, levosimendan, on haemodynamics, coronary blood flow and myocardial substrate utilization early after coronary artery bypass grafting. Eur Heart J 1998; 19: 660-668.
Moiseyev VS, Põ der P, Andrejevs N, et al. Safety and ef-ficacy of a novel calcium sensitiser, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction: Randomised, placebo-controlled, doubleblind study (RUSSLAN). Eur Heart J 2002; 23: 1422-1432.
Sonntag S, Opitz C, Wellnhofer E, et al. Effects of the calcium sensitizer levosimendan on stunned myocardium after percutaneous transluminal coronary angioplasty. Eur Heart J 2000; 21: 40.
Luotolahti M, Lammintausta O, Ukkonen H, et al. Levosimendan, a calcium sensitizer and potassium channel opener, is safe and improves left ventricular function in acute myocardial infarction. Circulation1998; 98(Suppl I): (I)105-(I)106.
Cleland JGF, Pennell DJ, Ray SG, et al. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure(CHRISTMAStrial): Randomised controlled trial. Lancet2003; 362: 14-21.
Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351(9118): 1755-1762.
Schulte K-L. 24 h anti-anginal and anti-ischaemic effects with once daily felodipine. A double-blind comparison with nifedipine, twice daily, and placebo in patients with stable exercise induced angina pectoris. Eur Heart J 1995; 16: 171-176.
Cohn JN, Ziesche S, Smith R, et al. Effect of the calcium antagonist felodipine as supplementary vasodilator therapy in patients with chronic heart failure treated with enalapril: VHeFT III. Vasodilator-Heart Failure Trial (V-HeFT) study group. Circulation 1997; 96(3): 856-863.
Weissler AM, Schoenfeld CD. Effect of digitalis on systolic time intervals in heart failure. Am JMed Sci 1970; 259: 4-20.
Karlsson M, Korkolainen T, Wikberg T. Automated analysis of levosimendan in human plasma by on-line dialysis and liquid chromatography. Biomed Chromatogr 1997; 11: 54-58.
Kivikko M, Antila S, Eha J, Lehtonen L, Pentikäinen PJ. Pharmacokinetics of levosimendan and its metabolites during and after a 24-hour continuous infusion in patients with severe heart failure. Int J Clin Pharmacol Ther 2002; 40: 465-471.
Tassani P, Schad H, HeimischW, et al. Effect of the calcium sensitizer levosimendan on the performance of ischaemic myocardium in anaesthetised pigs. Cardiovasc Drugs Ther 2002; 5: 435-441.
Pieske B. Levosimendan in regional myocardial ischemia. Cardiovasc Drugs Ther2002; 5: 379-381.
Scott MJ, Randolph PH, Leier CV. Reproducibility of systolic and diastolic time intervals in normal humans: An important issue in clinical cardiovascular pharmacology. J Cardiovasc Pharmacol 1989; 13: 125-130.
Li Q, Belz GG. Systolic time intervals in clinical pharmacology. Eur J Clin Pharmacol 1993; 44: 415-421.
Lilleberg J, Sundberg S, Häyhä M, Akkila J, Nieminen MS. Hemodynamic dose-efficacy of levosimendan in healthy volunteers. Eur J Clin Pharmacol 1994; 47: 267-274.
Sundberg S, Lilleberg J, Nieminen MS, Lehtonen L. Hemodynamic and neurohumoral effects of levosimendan, a new calcium sensitizer, at rest and during exercise in healthy men. Am J Cardiol 1995; 75: 1061-1066.
Hosenpud JD for the Oral Levosimendan Study Group. Levosimendan, a novel myofilament calcium sensitizer, allows weaning of parenteral inotropic therapy in patients with severe congestive heart failure. Am J Cardiol 1999; 83: 9(I)-11(I).
Kivikko M, Antila S, Eha J, Lehtonen L, Pentikäinen PJ. Pharmacodynamics and safety of a new calcium sensitizer, levosimendan and its metabolites during an extended infusion in patients with severe heart failure. J Clin Pharmacol 2002; 42: 43-51.
Takahashi R, Talukder MAH, Endoh M. Inotropic effects of OR-1896, an active metabolite of levosimendan, on canine ventricular myocardium. Eur J Pharmacol 2000; 400(1): 103-112.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Põder, P., Eha, J., Antila, S. et al. Pharmacodynamic Interactions of Levosimendan and Felodipine in Patients with Coronary Heart Disease. Cardiovasc Drugs Ther 17, 451–458 (2003). https://doi.org/10.1023/B:CARD.0000015860.08185.6d
Issue Date:
DOI: https://doi.org/10.1023/B:CARD.0000015860.08185.6d