Abstract
Objective: Arsenic is a known bladder carcinogen and populations exposed to high arsenic levels in their water supply have reported elevated bladder cancer mortality and incidence rates. To examine the effects of lower levels of arsenic exposure on bladder cancer incidence, we conducted a case–control study in New Hampshire, USA where levels above 10 μ/l are commonly found in private wells.
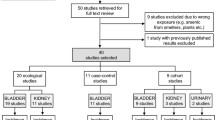
Methods: We studied 383 cases of transitional cell carcinoma of the bladder cancer, newly diagnosed between July 1, 1994 and June 30, 1998 and 641 general population controls. Individual exposure to arsenic was determined in toenail clippings using instrumental neutron activation analysis.
Results: Among smokers, an elevated odds ratio (OR) for bladder cancer was observed for the uppermost category of arsenic (OR: 2.17, 95% CI: 0.92–5.11 for greater than 0.330 mcg/g compared to less than 0.06 μ/g). Among never smokers, there was no association between arsenic and bladder cancer risk.
Conclusions: These, and other data, suggest that ingestion of low to moderate arsenic levels may affect bladder cancer incidence, and that cigarette smoking may act as a co-carcinogen.
Similar content being viewed by others
References
Hopenhayn-Rich C, Biggs ML, Fuchs A, et al. (1996) Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology 7: 117–124.
Chen CJ, Kuo TL, Wu MM (1988) Arsenic and cancers. Lancet 1: 414–415.
Rivara MI, Cebrian M, Corey G, Hernandez M, Romieu I (1997) Cancer risk in an arsenic-contaminated area of Chile. Toxicol Ind Health 13: 321–338.
Chiou HY, Hsueh YM, Liaw KF, et al. (1995) Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res 55: 1296–1300.
Tsuda T, Babazono A, Yamamoto E, et al. (1995) Ingested arsenic and internal cancer: a historical cohort study followed for 33 years. Am J Epidemiol 141: 198–209.
Cuzick J, Sasieni P, Evans S (1992) Ingested arsenic, keratoses, and bladder cancer. Am J Epidemiol 136: 417–421.
Bates MN, Smith AH, Cantor KP (1995) Case-control study of bladder cancer and arsenic in drinking water. Am J Epidemiol 141: 523–530.
Kurttio P, Pukkala E, Kahelin H, Auvinen A, Pekkanen J (1999) Arsenic concentrations in well water and risk of bladder and kidney cancer in Finland. Environ Health Perspect 107: 705–710.
Chiou HY, Chiou ST, Hsu YH, et al. (2001) Incidence of transitional cell carcinoma and arsenic in drinking water: a follow-up study of 8, 102 residents in an arseniasis-endemic area in northeastern Taiwan [comment]. Am J Epidemiol 153: 411–418.
Karagas MR, Tosteson TD, Blum J, et al. (1998) Design of an epidemiologic study of drinking water Arsenic exposure and skin and bladder cancer risk in a US population. Environ Health Perspect 106: 1047–1050.
Morris Brown L, Hoar Zahm S, Hoover RN, Fraumeni JF (1995) High bladder cancer mortality in rural New England (United States): an etiologic study. Cancer Causes Control 6: 361–368.
Karagas MR, Stukel TA, Tosteson TD (2002) Assessment of cancer risk and environmental levels of arsenic in New Hampshire. Int J Hyg Environ Health 205: 85–94.
Karagas MR, Stukel TA, Morris JS, et al. (2001) Skin cancer risk in relation to toenail arsenic concentrations in a US populationbased case-control study. Am J Epidemiol 153: 559–565.
Newcomb PA, Longnecker MP, Storer BE, et al. (1996) Long-term hormone replacement therapy and risk of breast cancer in postmenopausal women. Am J Epidemiol 142: 788–795.
Karagas MR, Tosteson TD, Blum J, et al. (2000) Measurement of low levels of arsenic exposure: a comparison of water and toenail concentrations. Am J Epidemiol 152: 84–90.
Nichols TA, Morris JS, Mason MM, et al. (1998) The study of human nails as an intake monitor for arsenic using neutron activation analysis. J Radioanal Nucl Chem 236: 51–56.
Altman NS (1992) An introduction to kernal and nearest-neighbor nonparametric regression. Am Stat 46: 175–185.
Breslow NE, Day NE (1980) Statistical Methods in Cancer Research. Volume 1-The Analysis of Case-control Studies Lyon: IARC.
Pastor R, Guallar E (1998) Use of two-segmented logistic regression to estimate change-points in epidemiologic studies. Am J Epidemiol 148: 631–642.
Tsai S-M, Wang T-N, Ko Y-C (1999) Mortality for certain diseases in areas with high levels of arsenic in drinking water. Arch Environ Health 54: 186–193.
Chen CJ, Chuang YC, Lin TM, Wu HY (1985) Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: high-arsenic artesian well water and cancers. Cancer Res 45: 5895–5899.
Chen CJ, Wang CJ (1990) Ecological correlation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer Res 50: 5470–5474.
Wu MM, Kuo TL, Hwang YH, Chen CJ (1989) Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol 130: 1123-1132.
Chiang HS, Guo HR, Hong CL, Lin SM, Lee EF (1993) The incidence of bladder cancer in the black foot endemic area in Taiwan. Br J Cancer 71: 272–278.
Guo HR, Chiang HS, Hu H, Lipsitz SR, Monson RR (1997) Arsenic in drinking water and incidence of urinary cancers. Epidemiology 8: 545–550.
Smith AH, Goycolea M, Haque R, Biggs ML (1998) Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol 147: 660–669.
Chen CJ, Chuang YC, You SL, Lin TM, Wu HY (1986) A retrospective study on malignant neoplasms of bladder, lung and liver in blackfoot disease endemic area in Taiwan. Br J Cancer 53: 399–405.
Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL (1999) Drinking water arsenic in Utah: a cohort mortality study [comment]. Environ Health Perspect 107: 359–365
Tosteson T, Karagas M (2000) Designing an arsenic bladder cancer case-control study: what sample size is needed to detect the beginning of a dose response? In: Centeno J, Collery P, Vernet G, Finnkelman R, Gibb H, Etienne J, eds., Metal Ions in Biology and Medicine, Vol. 6, Paris: John Libbey and Company Ltd., pp. 28–30.
Wiencke JK, Yager JW, Varkonyi A, Hultner M, Lutze LH (1997) Study of arsenic mutagenesis using the plasmid shuttle vector pZ189 propagated in DNA repair proficient human cells. Mutat Res 386: 335–344.
Tran HP, Prakash AS, Barnard R, Chiswell B, Ng JC (2002) Arsenic inhibits the repair of DNA damage induced by benzo(a)pyrene. Toxicol Lett 133: 59–67.
Li JM, Rossman TG (1989) Inhibition of DNA ligase activity by arsenite: a possible mechanism of its comutagenesis. Mol Toxicol 2: 1–9.
Yager JW, Wiencke JK (1997) Inhibition of poly(ADP-ribose) polymerase by arsenite. Mutat Res 386: 345–351.
Andrew AS, Karagas MR, Schned A, et al. (2003) Decreased expression of DNA repair genes ERCC1, XPF, and XPB, but not XPG or XPA among individuals exposed to arsenic in drinking water. Int J Cancer 104: 263–268.
Zakharyan RA, Sampayo-Reyes A, Healy SM, et al. (2001) Human monomethylarsonic acid (MMA(V)) reductase is a member of the glutathione-S-transferase superfamily. Chem Res Toxicol 14: 1051–1057.
Vahter M (2000) Genetic polymorphism in the biotransformation of inorganic arsenic and its role intoxicity. Toxicol Lett 112-113: 207–217.
Chen Y-C, Su H-JJ, Guo Y-LL, et al. (2003) Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control 14: 303–310.
Garland M, Morris JS, Rosner BA, et al. (1993) Toenail trace element levels as biomarkers: reproducibility over a 6-year period [published erratum appears in Cancer Epidemiol Biomarkers Prev 1994 Sep; 3(6): 523]. Cancer Epidemiol Biomarkers Prev 2: 493–497.
Karagas MR, Le XC, Morris S, et al. (2001) Markers of low level arsenic exposure for evaluating human cancer risk in a US population. Int J Occup Environ Health 14: 171–175.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karagas, M.R., Tosteson, T.D., Morris, J.S. et al. Incidence of Transitional Cell Carcinoma of the Bladder and Arsenic Exposure in New Hampshire. Cancer Causes Control 15, 465–472 (2004). https://doi.org/10.1023/B:CACO.0000036452.55199.a3
Issue Date:
DOI: https://doi.org/10.1023/B:CACO.0000036452.55199.a3