Abstract
Psychosocial factors have been described as affecting cellular immune measures in healthy subjects. In patients with early breast cancer we explored bi-directional psycho-immune effects to determine whether subjective burden has an impact on immune measures, and vice versa.
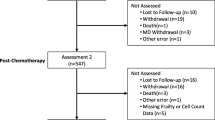
Patients (n= 239) operated for early breast cancer and randomized into International Breast Cancer Study Group (IBCSG) adjuvant clinical trials were assessed immediately before the beginning of adjuvant treatment (baseline) and 3 and 6 months thereafter, at the beginning of the corresponding treatment cycle. Cellular immune measures (leukocytes, lymphocytes, lymphocyte subset counts), markers of activation of the cellular immune system (β 2-microglobulin, soluble interleukin–2 receptor serum levels), and self-report subjective burden (global indicators of physical well-being, mood, coping effort) were assessed concurrently. The relationship between subjective burden and gradients of immune measures was investigated with regression analyses controlling for adjuvant treatment. There was a pattern of small negative associations between all variables assessing subjective burden before the beginning of adjuvant therapy with the gradients of the markers of activation of the cellular immune system and NK cell counts. In particular, better mood predicted a decline in the course of β 2-microglobulin and IL-2r at months 3 and 6. The gradient of β 2-microglobulin was associated with mood and coping effort at month 3. However, the effect sizes were very small. In conclusion, in this explorative investigation, there was an indication for subjective burden affecting and being affected by markers of activation of the cellular immune system during the first 3 and 6months of adjuvant therapy. The question of clinical significance remains unanswered. These associations have to be investigated with refined assessment tools and schedules.
Similar content being viewed by others
References
Herbert TB, Cohen S: Stress and Immunity in Humans: A Meta-Analytic Review. Psychosom Med 55: 364–379, 1993
Garssen B, Goodkin K: On the role of immunological factors as mediators between psychosocial factors and cancer progression. Psych Res 85: 51–61, 1999
Cohen S, Rabin BS: Psychologic stress, immunity, and cancer. J Natl Cancer Inst 90: 3–4, 1998
Mitchel RJ: The delayed hypersensitivity response in primary breast carcinoma as an index of host resistance. Br J Surg 59: 505–508, 1972
Roberts MM, Jones Williams W: The delayed hypersensi-tivity reaction in breast cancer. Br J Surg 61: 522–549, 1974
Nemoto T, Han T, Minowada J, Angkur V, Chamberlain A, Dao TL: Cell-mediated immune status of breast cancer patients: Evaluation by skin tests, lymphocyte stimulation, and counts of rosette-forming cells. J Natl Cancer Inst 53: 641–671, 1974
Catalona WJ, Sample WF, Chretien PB: Lymphocyte reactivity in cancer patients: Correlation with tumor histology and clinical stage. Cancer 31: 65–71, 1973
Head JF, Elliot RL, McCoy JL: Evaluation of lymphocyte immunity in breast cancer patients. Breast Cancer Res Treat 26: 77–88, 1993
Sabbioni MEE, Siegrist HP, Bacchi M, et al: Association between immunity and prognostic factors in early stage breast cancer patients before adjuvant treatment. Breast Cancer Res Treat 59: 279–287, 2000
Garner WL, Minton JP, James AG, Hoffmann CC: Human breast cancer and impaired NK cell function. J Surg Oncol 24: 64–66, 1983
Kiecolt JK, Glaser R. Psychoneuroimmunology and can-cer: fact or ction? Eur J Cancer 35: 1603–1607, 1999
Hürny C, Bernhard J, Coates AS, et al: Impact of adjuvant therapy on QOL in women with node-positive breast cancer. Lancet 347: 1279–1284, 1996
van der Pompe G, Antoni M, Visser A, Garssen B: Adjustment to breast cancer: The psychobiological effects of psychosocial interventions. Pat Educ and Couns 28: 209–219, 1996
Lekander M, Fürst CJ, Rotstein S, Blomgren H, Fredrik-son M: Social support and immune status during and after chemotherapy for breast cancer. Acta Oncol 35: 31–37, 1996
Tjemsland L, Soreide JA, Matre R, Malt UF: Preoperative psychological variables predict immunologic status in patients with operable breast cancer. Psycho-Oncol;6: 311–320, 1997
Andersen BL, Farrar WB, Golden-Kreutz D, et al: Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst 67: 30–36, 1998
Levy SM, Herberman RB, Maluish AM, Schlien G, Lippman M: Prognostic risk assessment in primary breast cancer by behavioral and immunological parameters. Health Psychol 4: 99–113, 1985
Levy S, Herberman R, Lippman M, d 'Angelo T: Correla-tion of stress factors with sustained depression of natural killer cell activity and predicted prognosis in patients with breast cancer. J Clin Oncol 5: 348–353, 1987
Marsland AL, Manuck SB, Fazzari TV, Stewart CJ, Rabin BS: Stability of individual differences in cellular immune responses to acute psychological stress. Psychosom Med 57: 295–298, 1995
International Breast Cancer Study Group: Duration and reintroduction of adjuvant chemotherapy for nodes-posi-tive premenopausal breast cancer patients. J Clin Oncol 14: 1885–1894, 1996
International Breast Camcer Study Group: Effectiveness of adjuvant chemotherapy in combination with tamoxifen for node-positive postmenopausal breast cancer patients. J Clin Oncol 15: 1385–1394, 1997
Bernhard J, Sullivan M, Hürny C, Coates AS, Rudenstam CM: Clinical relevance of single item quality of life indicators in cancer clinical trials. Br J Cancer 84(9): 1156–1165, 2001
Butow P, Coates A, Dunn S, Bernhard J, Hürny C: On the receiving end IV: Validation of quality of life indicators. Ann Oncol 2: 597–603, 1991
Coates A, Glasziou P, McNeil D: Measurement of quality of life during cancer chemotherapy. Annals Oncol 1: 213–217, 1990
Hürny C, Bernhard J, Gelber RD, et al: Quality of life measures for patients receiving adjuvant therapy for breast cancer: an International Trial. Eur J Cancer 28: 118–124, 1992
Coates A, Gebski V, Bishop JF, et al: Improving the quality of life during chemotherapy for advanced breast cancer. N Engl J Med 317: 1490–1495, 1987
Bernhard J, Castiglione-Gertsch M, Schmitz S-FH, et al: Quality of life in postmenopausal patients with breast cancer after failure of tamoxifen: formestane versus meges-trol acetate as second-line hormonal treatment. Eur J Cancer 35: 913–920, 1999
Bernhard J, Thürlimann B, Schmitz S-FH, et al: Defining clinical bene t in postmenopausal patients with breast cancer under second-line endocrine treatment: does quality of life matter? J Clin Oncol 17: 1672–1679, 1999
Coates AS, Hürny C, Peterson HF, et al: Quality-of-life scores predict outcome in metastatic but not early breast cancer. J Clin Oncol 16: 3768–3774, 2000
Coates AS, Gebski V, Signorini D, et al: Prognostic value of quality-of-life scores during chemotherapy for advanced breast cancer.Australian New Zealand Breast Cancer Trials Group. J Clin Oncol 10: 1833–1838, 1992
Coates AS, Thomson D, McLeod GR, et al: Prognostic value of quality of life scores in a trial of chemotherapy with or without interferon in patients with metastatic melanoma. Eur J Cancer 29A: 1731–1734, 1993
Herschbach P, Marten-Mittag B, Henrich G: Revision und Prüfung des Fragebogens zur Belastung von Krebskranken (FBK-R23). Zeitschft Med Psychol 12: 69–76, 2003
Gerits P, De Brabander B: Psychosocial predictors of psychological, neurochemical, and immunological symp-toms of acute stress among breast cancer patients. Psych Res 85: 95–103, 1999
Gruber BL, Hersh SP, Hall NRS, et al: Immunological responses of breast cancer patients to behavioral interven-tions. Biofeedback Self-Regul 18: 1–22, 1993
Levy MS, Herberman RB, Lippman M, D 'Angelo T, Lee J: Immunological and psychosocial predictors of disease recurrence in patients with early-stage breast cancer. Behav Med 17: 67–75, 1991
Kiecolt-Glaser JK, Glaser R: Psychoneuroimmunology and health consequences: data and shared mechanisms. Psycho-som Med 57: 269–274, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sabbioni, M.E., Bernhard, J., Siegrist, HP. et al. Does Subjective Burden of Early Breast Cancer and its Treatment Affect Immune Measures During Adjuvant Therapy?. Breast Cancer Res Treat 87, 75–86 (2004). https://doi.org/10.1023/B:BREA.0000041584.53863.a7
Issue Date:
DOI: https://doi.org/10.1023/B:BREA.0000041584.53863.a7