Abstract
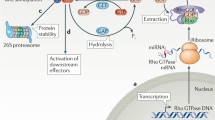
Mitogenic growth factor- and integrin-dependent signaling pathways cooperate to control the proliferation of nontransformed cells. As integral mediators of these networks, the Rho family of GTPases play a pivotal role in G1 cell cycle progression, primarily through regulation of cyclin D1 expression, as well as the levels of the cyclin-dependent kinase inhibitors p21cip1 and p27kip1. Such dual control of both the critical positive and negative regulators of G1 progression make the Rho GTPases prime candidates to target the autonomous proliferation which typifies cancer cells. Cyclin D1 has been identified as an important oncogene and the cdk inhibitors as tumor suppressors in human breast carcinogenesis. Evidence pointing to the potential role of Rho-dependent pathways and their interaction with oncogenic Ras in contributing to such cell cycle abnormalities that characterize human breast cancer is also presented.
Similar content being viewed by others
References
Sherr CJ, Roberts JM: CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13: 1501–1512, 1999
LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E: New functional activities for the p21 family of CDK inhibitors. Genes Dev 11: 847–862, 1997
Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ: The p21Cip1 and p27Kip1 CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J 18: 1571–1583, 1999
Weinberg R: The retinoblastoma protein and cell cycle control. Cell 81: 323–330, 1995
Degregori J, Kowalik T, Nevins JR: Cellular targets for activation by the E2F1 transcription factor include DNA synthesis-and G1/S-regulatory genes. Mol Cell Biol 15: 4215–4224, 1995
Pardee A: G1 events and regulation of cell proliferation. Science 246: 603–608, 1989
Assoian RK: Anchorage-dependent cell cycle progression. J Cell Biol 136: 1–4, 1997
Assoian RK, Schwartz MA: Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci 114: 2553–2560, 2001
Lin TH, Chen Q, Howe A, Juliano RL: Cell anchorage permits efficient signal transduction between Ras and its downstream kinases. J Biol Chem 272: 8849–8852, 1997
Renshaw MW, Ren X-D, Schwartz MA: Growth factor activation of MAP kinase requires cell adhesion. EMBO J 16: 5592–5599, 1997
Weber JD, Raben DM, Phillips PJ, Baldessare J: Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J 326: 61–68, 1997
Roovers K, Davey G, Zhu X, Bottazzi ME, Assoian RK: ±5β1 integrin controls cyclin D1 expression by sustaining mitogenactivated protein kinase activity in growth factor-treated cells. Mol Biol Cell 10: 3197–3204, 1999
Aplin AE, Stewart SA, Assoian RK, Juliano RL: Integrinmediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J Cell Biol 153: 273–281, 2001
Zhu X, Ohtsubo M, Bohmer RM, Roberts JM, Assoian RK: Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-CDK2, and phosphorylation of the retinoblastoma protein. J Cell Biol 133: 391–403, 1996
Bohmer RM, Scharf E, Assoian RK: Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin D1. Mol Biol Cell 7: 101–111, 1996
Schulze A, Zerfass-Thome K, Berges J, Middendorp S, Jansen-Durr P, Henglein B: Anchorage-dependent transcription of the cyclin A gene. Mol Cell Biol 16: 4632–4638, 1996
Resnitzky D: Ectopic expression of cyclin D1 but not cyclin E induces anchorage-independent cell cycle progression. Mol Cell Biol 17: 5640–5647, 1997
Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E: Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science 271: 499–502, 1996
Hansen LK, Mooney DJ, Vacanti JP, Ingber DE: Integrin binding and cell spreading on extracellular matrix act at different points in the cell cycle to promote hepatocyte growth. Mol Biol Cell 5: 967–975, 1994
Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE: Geometric control of cell life and death. Science 276: 1425–1428, 1997
Huang S, Chen CS, Ingber DE: Control of cyclin D1, p27Kip1, and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell 9: 3179–3193, 1998
Clark EA, King WG, Brugge JS, Symons M, Hynes RO: Integrin-mediated signals regulated by members of the Rho family GTPases. J Cell Biol 142: 573–586, 1998
Renshaw MW, Toksoz D, Schwartz MA: Involvement of the small GTPase Rho in integrin-mediated activation of mitogenactivated protein kinase. J Biol Chem 271: 21691–21694, 1996
Price LS, Leng J, Schwartz MA, Bokoch GM: Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell 9: 1863–1871, 1998
Schwartz MA, Toksoz D, Khosravi-Far R: Transformation by Rho exchange factor oncogenes is mediated by activation of an integrin-dependent pathway. EMBO J 15: 6525–6530, 1996
Ren X-D, Kiosses WB, Schwartz MA: Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J 18: 578–585, 1999
del Pozo MA, Price LS, Alderson NB, Ren X-D, Schwartz MA: Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J 19: 2008–2014, 2000
Olson MF, Ashworth A, Hall A: An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science 269: 1270–1272, 1995
Westwick JK, Lambert QT, Clark GJ, Symons M, Van Aelst L, Pestell RG, Der CJ: Rac regulation of transformation, gene expression, and actin organization by multiple PAK-independent pathways. Mol Cell Biol 17: 1324–1335, 1997
Gille H, Downward J: Multiple Ras effector pathways contribute to G1 cell cycle progression. J Biol Chem 274: 22033–22040, 1999
Welsh CF, Roovers K, Villanueva J, Liu Y, Schwartz MA, Assoian RK: Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nature Cell Biol 3: 950–957, 2001
Gjoerup O, Lukas J, Bartek J, Willumsen BM: Rac and Cdc42 are potent stimulators of E2F-dependent transcription capable of promoting retinoblastoma susceptibility gene product hyperphosphorylation. J Biol Chem 273: 18812–18818, 1998
Page K, Li J, Hodge JA, Liu PT, Vanden Hoek TL, Becker LB, Pestell RG, Rosner MR, Hershenson MB: Characterization of a Rac1 signaling pathway to cyclin D1 expression in airway smooth muscle cells. J Biol Chem 274: 22065–22071, 1999
Joyce D, Bouzahzah B, Fu M, Albanese C, D'Amico M, Steer J, Klein JU, Lee RJ, Segall JE, Westwick JK, Der CJ, Pestell RG: Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-κ-dependent pathway. J Biol Chem 274: 25245–25249, 1999
Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, Giancotti FG: Integrin-specific activation of Rac controls progression through the G1 phase of the cell cycle. Mol Cell 8: 115–127, 2001
Weber JD, Hu W, Jefcoat SC, Raben DM, Baldassare JJ: Ras-stimulated extracellular signal-related kinase 1 and RhoA activities coordinate platelet-derived growth factor-induced G1 progression through the independent regulation of cyclin D1 and p27Kip1. J Biol Chem 272: 32966–32971, 1997
Danen EHJ, Sonnenveld P, Sonnenberg A, Yamada KM: Dual stimulation of Ras/mitogen-activated protein kinase and RhoA by cell adhesion to fibronectin supports growth factorstimulated cell cycle progression. J Cell Biol 151: 1413–1422, 2000
Hansen LK, Albrecht JH: Regulation of the hepatocyte cell cycle by type I collagen matrix: role of cyclin D1. J Cell Sci 112: 2971–2981, 1999
Hengst L, Reed SI: Translational control of p27Kip1accumulation during the cell cycle. Science 271: 1861–1864, 1996
Hirai A, Nakamura S, Noguchi Y, Yasuda T, Kitagawa M, Tatsuno I, Oeda T, Tahara K, Terano T, Narumiya S, Kohn LD, Saito Y: Geranyleranylated Rho small GTPase(s) are essential for the degradation of p27Kip1and facilitate the progression from G1 to S phase in growth-stimulated rat FRTL-5 cells. J Biol Chem 272: 13–16, 1997
Vogt A, Sun J, Qian Y, Hamilton AD, Sebti SM: The geranylgeranyltransferase-I inhibitor GGTI-298 arrests human tumor cells in G0/G1 and induces p21WAF1/CIP1/SDI1in a p53-independent manner. J Biol Chem 272: 27224–27229, 1997
Auer KL, Park J-S, Seth P, Coffey RJ, Darlington G, Abo A, McMahon M, DePinho RA, Fisher PB, Dent P: Prolonged activation of the mitogen-activated protein kinase pathway promotes DNA synthesis in primary hepatocytes from p21Cip-1/WaF1-null mice, but not in hepatocytes from p16INK4a-null mice. Biochem J 336: 551–560, 1998
Adnane J, Bizouarn FA, Qian Y, Hamilton AD, Sebti SM: p21WAF1/CIP1is upregulated by the geranylgeranyltransferase I inhibitor GGTI-298 through a transforming growth factor β-and Sp1-responsive element: involvement of the small GTPase RhoA. Mol Cell Biol 18: 6962–6970, 1998
Olson MF, Paterson HF, Marshall CJ: Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature 394: 295–298, 1998
Bottazzi ME, Zhu X, Bohmer RM, Assoian RK: Regulation of p21cip1expression by growth factors and the extracellular matrix reveals a role for transient ERK activity in G1 phase. J Cell Biol 146: 1255–1264, 1999
Millard SS, Vidal A, Markus M, Koff A: A U-rich element in the 5′ untranslated region is necessary for the translation of p27 mRNA. Mol Cell Biol 20: 5947–5959, 2000
Agrawal D, Hauser P, McPherson F, Dong F, Garcia A, Pledger WJ: Repression of p27kip1synthesis by plateletderived growth factor in BALB/c3T3 cells. Mol Cell Biol 16: 4327–4336, 1996
Medema RH, Kops GJPL, Bos JL, Burgering BMT: AFX-like forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404: 782–787, 2000
Pagano M, Tam SW, Theodoras AM, Beer-Romano P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M: Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269: 682–685, 1995
Laufs U, Marra D, Node K, Liao JK: 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing Rho GTPaseinduced down-regulation of p27Kip1. J Biol Chem 274: 21926–21931, 1999
Hu W, Bellone CJ, Baldassare JJ: RhoA stimulates p27Kipdegradation through its regulation of cyclin E/CDK2 activity. J Biol Chem 274: 3396–3401, 1999
Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE: Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev 11: 1464–1478, 1997
Vidal A, Millard SS, Miller JP, Koff A: Rho activity can alter the translation of p27 mRNA and is important for RasV12-induced transformation in a manner dependent on p27 status. JBC 277: 16433–16440, 2002
Malek NP, Sundberg H, McGrew S, Nakayama K, Kyriakidis TR, Roberts JM: A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1in G1 and S phase. Nature 413: 323–327, 2001
Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K: Formation of actin stress fibers and focal adhesions enhanced by rho-kinase. Science 275: 1308–1311, 1997
Sahai E, Ishizaki T, Narumiya S, Treisman R: Transformation mediated by RhoA requires activity of ROCK kinases. Current Biol 9: 136–145, 1999
Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, Inoue M, Masatsugu K, Saito T, Fukunaga Y, Sakaguchi S, Arai H, Ohno N, Komeda M, Nakao K: Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation 101: 2030–2033, 2000
Iwamoto H, Nakamuta M, Tada S, Sugimoto R, Enjoji M, Nawata H: A p160ROCK-specific inhibitor, Y-27632, attenuates rat hepatic stellate cell growth. J Hepatol 32: 762–770, 2000
Sahai E, Olson MF, Marshall CJ: Cross-talk between Ras and Rho signaling pathways in transformation favours proliferation and increase motility. EMBO J 20: 755–766, 2001
Aktories K, Just I: Monoglucosylation of low-molecular mass GTP-binding Rho proteins by clostridial cytotoxins. Trends Cell Biol 5: 441–443, 1995
Lammie GA, Fantl V, Smith R, Shuuring E, Brookes S, Michalides R, Dickson C, Arnold A, Peters G: D11S287, a putative oncogene on chromosome 11q13, is amplified and expressed in squamous cell and mammary carcinomas and linked to BCL-1. Oncogene 6: 439–444, 1991
Schuuring E, Verhoeven E, Mooi WJ, Michalides RJAM: Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene 7: 355–361, 1992
Theillet C, Adane J, Szepetowski P, Simon M-P, Jeanteur P, Birnbaum D, Gaudray P: BCL-1 participates in the 11q13 amplification found in breast cancer. Oncogene 5: 147–149, 1990
Bartkova J, Lukas J, Muller H, Lutzhoft D, Strauss M, Bartek J: Cyclin D1 protein expression and function in human breast cancer. Int J Cancer 57: 353–361, 1994
Buckley MF, Sweeney KJE, Hamilton JA, Sini RL, Manning DL, Nicholson RI, deFazio A, Watts CKW, Musgrove EA, Sutherland RL: Expression and amplification of cyclin genes in human breast cancer. Oncogene 8: 2127–2133, 1993
Gillett C, Smith P, Gregory W, Richards M, Millis R, Peters G, Barnes D: Cyclin D1 and prognosis in human breast cancer. Int J Cancer 69: 92–99, 1996
Weistat-Saslow D, Merino MJ, Manrow RE, Lawrence JA, Bluth RF, Wittenbel KD, Simpson JF, Page DL, Steeg PS: Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nature Med 1: 1257–1260, 1995
Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV: Mammary hyperplasia and carcinoma in MMTVcyclin D1 transgenic mice. Nature 369: 669–671, 1994
Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C: Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev 9: 2364–2372, 1995
Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA: Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82: 621–630, 1995
Yu Q, Geng Y, Sicinski P: Specific protection against breast cancers by cyclin D1 ablation. Nature 411: 1017–1021, 2001
Fredersdorf S, Burns J, Milne AM, Packham G, Fallis L, Gillett CE, Royds JA, Peston D, Hall PA, Hanby AM, Barnes DM, Shousha S, O'Hare MJ, Lu X: High level expression of p27kip1 and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27kip1and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci 94: 6380–6385, 1997
Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR, Roberts JM: Expression of cell-cycle regulators p27Kip1and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nature Med 3: 222–225, 1997
Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard KI, Slingerland JM: Decreased levels of the cell-cycle inhibitor p27Kip1protein: prognostic implications in primary breast cancer. Nature Med 3: 227–230, 1997
Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M: Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nature Med 3: 231–234, 1997
Ferrando AA, Balbin M, Pendas AM, Vizoso F, Velasco G, Lopez-Otin C: Mutational analysis of the human cyclindependent kinase inhibitor p27kip1in primary breast carcinomas. Hum Genet 97: 91–94, 1996
Jiang M, Shao Z-M, Wu J, Lu J-S, Yu L-M, Yuan J-D, Han Q-X, Shen Z-Z, Fontana JA: p21/waf1/cip1 and mdm-2 expression in breast carcinoma patients as related to prognosis. Int J Cancer 74: 529–534, 1997
Shen Z, Wen X-F, Lan F, Shen Z-Z, Shao Z-M: The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin Cancer Res 8: 2085–2090, 2002
Adnane J, Jackson RJ, Nicosia SV, Cantor AB, Pledger WJ, Sebti SM: Loss of p21WAF1/CIP1 accelerates Ras oncogenesis in a transgenic/knockout mammary cancer model. Oncogene 19: 5338–5347, 2000
Bearss DJ, Lee RJ, Troyer DA, Pestell RG, Windle JJ: Differential effects of p21(WAF1/CIP1) deficiency on MMTV-ras and MMTV-myc mammary tumor properties. Cancer Res 62: 2077–2084, 2002
Liberto M, Cobrinik D, Minden A: Rho regulates p21CIP1, cyclin D1, and checkpoint control inmammary epithelial cells. Oncogene 21: 1590–1599, 2002
Mira J-P, Benard V, Groffen J, Sanders LC, Knaus UG: Endogenous hyperactive rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc Natl Acad Sci 97: 185–189, 2000
van Golen KL, Davies S, Wu ZF, Wang YF, Bucana CD, Root H, Chandrasekharappa S, Strawderman M, Ethier SP, Merajver SD: A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and rhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res 5: 2511–2519, 1999
Fritz G, Just I, Kaina B: Rho GTPases are overexpressed in human tumors. Int J Cancer 81: 682–687, 1999
Schnelzer A, Prechtzel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E: Rac1 in human breast cancer: overexpression, mutational analysis, and characterization of a new isoform, Rac1b. Oncogene 19: 3013–3020, 2000
van Golen KL, Wu Z-F, Qiao XT, Bao LW, Merajver SD: RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res 60: 5832–5838, 2000
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Welsh, C.F. Rho GTPases as Key Transducers of Proliferative Signals in G1 Cell Cycle Regulation. Breast Cancer Res Treat 84, 33–42 (2004). https://doi.org/10.1023/B:BREA.0000018425.31633.07
Issue Date:
DOI: https://doi.org/10.1023/B:BREA.0000018425.31633.07