Abstract
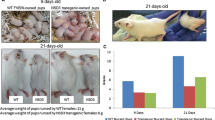
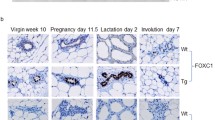
A rat strain carrying the human c-Ha-ras protooncogene, established by our laboratory, is highly susceptible to mammary chemical carcinogens. The transgenic rats exhibit increased number of terminal endbuds (TEBs) at the tips of developing ducts in the mammary gland compared to non-transgenic littermates. Confocal microscopy revealed the level of active mitogen-activated protein kinase to be elevated in these TEBs, and a close correlation between their numbers and tumorigenic response initiated by 7,12-dimethylbenz[a]anthracene was confirmed. Single injections of N-methyl-N-nitrosourea into the transgenic rats caused mutations in codon 12 of human c-Ha-ras transgene in TEBs before tumor development, supporting the conclusion that these structures are the major targets of chemical carcinogens. In contrast, with spontaneous development of lesions, alveolar hyperplasia with elevated expression levels of rat and human c-Ha-ras protooncogenes is the first morphological alteration which becomes apparent. Some but not all hyperplastic alveolar nodules were found to harbor mutations in the transgene. The results indicate that elevated expression of c-Ha-ras protooncogene is sufficient in itself to cause a highly proliferative phenotype of mammary alveoli. Our data suggest that TEBs and acini are the major targets for chemical and sporadic carcinogenesis, respectively, in the mammary glands of human c-Ha-ras protooncogene transgenic rats.
Similar content being viewed by others
References
Russo J, Wilgus G, Russo IH: Susceptibility of the mammary gland to carcinogenesis: I Differentiation of the mammary gland as determinant of tumor incidence and type of lesion. Am J Pathol 96: 721–736, 1979
Russo J, Russo IH: Experimentally induced mammary tumors in rats. Breast Cancer Res Treat 39: 7–20, 1996
Sukumar S, McKenzie K, Chen Y: Animal models for breast cancer. Mutat Res 333: 37–44, 1995
Thompson HJ, McGinley JN, Rothhammer K, Singh M: Rapid induction of mammary intraductal proliferations, ductal carcinoma in situ and carcinomas by the injection of sexually immature female rats with 1-methyl-1-nitrosourea. Carcinogenesis 16: 2407–2411, 1995
Thompson HJ, McGinley JN, Wolfe P, Singh M, Steele VE, Kelloff GJ: Temporal sequence of mammary intraductal proliferations, ductal carcinomas in situ and adenocarcinomas induced by 1-methyl-1-nitrosourea in rats. Carcinogenesis 19: 2181–2185, 1998
Russo J, Russo IH: Biological and molecular bases of mammary carcinogenesis. Lab Invest 57: 112–137, 1987
Humphreys RC: Programmed cell death in the terminal endbud. J Mammary Gland Biol Neoplasia 4: 213–220, 1999
Schuller HM: Mechanisms of smoking-related lung and pancreatic adenocarcinoma development. Nat Rev Cancer 2: 455–463, 2002
Grady WM, Markowitz SD: Genetic and epigenetic alterations in colon cancer. Annu Rev Genomics Hum Genet 3: 101–128, 2002
Sukumar S, Notario V, Martin-Zanca D, Barbacid M: Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature 306: 658–661, 1983
Leon J, Kamino H, Steinberg JJ, Pellicer A: H-ras activation in benign and self-regressing skin tumors (keratoacanthomas) in both humans and an animal model system. Mol Cell Biol 8: 786–793, 1988
Asamoto M, Ochiya T, Toriyama-Baba H, Ota T, Sekiya T, Terada M, Tsuda H: Transgenic rats carrying human c-Ha-ras proto-oncogenes are highly susceptible to N-methyl-N-nitrosourea mammary carcinogenesis. Carcinogenesis 21: 243–249, 2000
Kumar R, Sukumar S, Barbacid M: Activation of ras oncogenes preceding the onset of neoplasia. Science 248: 1101–1114, 1990
Filmus J, Robles AI, Shi W, Wong MJ, Colombo LL, Conti CJ: Induction of cyclin D1 overexpression by activated ras. Oncogene 9: 3627–3633, 1994
Reuther GW, Der CJ: The Ras branch of small GTPases: ras family members don't fall far from the tree. Curr Opin Cell Biol 12: 157–165, 2000
Miyakis S, Sourvinos G, Spandidos DA: Differential expression and mutation of the ras family genes in human breast cancer. Biochem Biophys Res Commun 251: 609–612, 1998
Bieche I, Lidereau R: Genetic alterations in breast cancer. Genes Chromosomes Cancer 14: 227–251, 1995
Knepper JE, Kittrell FS, Medina D, Butel JS: Spontaneous progression of hyperplastic outgrowths of the D1 lineage to mammary tumors: expression of mouse mammary tumor virus and cellular proto-oncogenes. Mol Carcinog 1: 229–238, 1989
Salh B, Marotta A, Matthewson C, Ahluwalia M, Flint J, Owen D, Pelech S: Investigation of the Mek-MAP kinase-Rsk pathway in human breast cancer. Anticancer Res 19: 731–740, 1999
von Lintig FC, Dreilinger AD, Varki NM, Wallace AM, Casteel DE, Boss GR: Ras activation in human breast cancer. Breast Cancer Res Treat 62: 51–62, 2000
Andres AC, Schonenberger CA, Groner B, Hennighausen L, LeMeur M, Gerlinger P: Ha-ras oncogene expression directed by a milk protein gene promoter: tissue specificity, hormonal regulation, and tumor induction in transgenic mice. Proc Natl Acad Sci USA 84: 1299–1303, 1987
Thompson TA, Kim K, Gould MN: Harvey ras results in a higher frequency of mammary carcinomas than Kirsten ras after direct retroviral transfer into the rat mammary gland. Cancer Res 58: 5097–5104, 1998
Yu Q, Geng Y, Sicinski P: Specific protection against breast cancers by cyclin D1 ablation. Nature 411: 1017–1021, 2001
Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G: Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 385: 544–548, 1997
Peli J, Schroter M, Rudaz C, Hahne M, Meyer C, Reichmann E, Tschopp J: Oncogenic Ras inhibits Fas ligand-mediated apoptosis by downregulating the expression of Fas. Embo J 18: 1824–1831, 1999
Kazama H, Yonehara S: Oncogenic K-Ras and basic fibroblast growth factor prevent Fas-mediated apoptosis in fibroblasts through activation of mitogen-activated protein kinase. J Cell Biol 148: 557–566, 2000
Ries S, Biederer C, Woods D, Shifman O, Shirasawa S, Sasazuki T, McMahon M, Oren M, McCormick F: Opposing effects of Ras on p53: transcriptional activation of mdm2 and induction of p19ARF. Cell 103: 321–330, 2000
Ota T, Asamoto M, Toriyama-Baba H, Yamamoto F, Matsuoka Y, Ochiya T, Sekiya T, Terada M, Akaza H, Tsuda H: Transgenic rats carrying copies of the human c-Ha-ras proto-oncogene exhibit enhanced susceptibility to N-butyl-N-(4-hydroxybutyl)nitrosamine bladder carcinogenesis. Carcinogenesis 21: 1391–1396, 2000
Tsuda H, Asamoto M, Ochiya T, Toriyama-Baba H, Naito A, Ota T, Sekiya T, Terada M: High susceptibility of transgenic rats carrying the human c-Ha-ras proto-oncogene to chemically-induced mammary carcinogenesis. Mutat Res 477: 173–182, 2001
Matsuoka Y: Rapid emergence of mammary preneoplastic and malignant lesions in human c-Ha-ras proto-oncogene transgenic rats: possible application for screening of chemopreventive agents. Toxicol Pathol (in press)
Han BS, Fukamachi K, Takasuka N, Ohnishi T, Maeda M, Yamasaki T, Tsuda H: Inhibitory effects of 17beta-estradiol and 4-n-octylphenol on 7,12-dimethylbenz[a]anthracene-induced mammary tumor development in human c-Ha-ras proto-oncogene transgenic rats. Carcinogenesis 23: 1209–1215, 2002
Matsuoka Y, Li X, Bennett V: Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol 142: 485–497, 1998
Fukamachi K, Matsuoka Y, Ohno H, Hamaguchi T, Tsuda H: Neuronal leucine-rich repeat protein-3 amplifies MAPK activation by epidermal growth factor through a carboxyl-terminal region containing endocytosis motifs. J Biol Chem 277: 43549–43552, 2002
Fukamachi K, Matsuoka Y, Kitanaka C, Kuchino Y, Tsuda H: Rat neuronal leucine-rich repeat protein-3: cloning and regulation of the gene expression. Biochem Biophys Res Commun 287: 257–263, 2001
Matsuda Y, Chapman VM: Application of fluorescence in situ hybridization in genome analysis of the mouse. Electrophoresis 16: 261–272, 1995
Bos JL: Ras oncogenes in human cancer: a review. Cancer Res 49: 4682–4689, 1989
Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell RG: Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem 270: 23589–23597, 1995
Liu JJ, Chao JR, Jiang MC, Ng SY, Yen JJ, Yang-Yen HF: Ras transformation results in an elevated level of cyclin D1 and acceleration of G1 progression in NIH 3T3 cells. Mol Cell Biol 15: 3654–3663, 1995
Lavoie JN, L'Allemain G, Brunet A, Muller R, Pouyssegur J: Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem 271: 20608–20616, 1996
Steeg PS, Zhou Q: Cyclins and breast cancer. Breast Cancer Res Treat 52: 17–28, 1998
Bartkova J, Lukas J, Muller H, Lutzhoft D, Strauss M, Bartek J: Cyclin D1 protein expression and function in human breast cancer. Int J Cancer 57: 353–361, 1994
Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G: Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res 54: 1812–1817, 1994
Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV: Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369: 669–671, 1994
Davies BR, Platt-Higgins AM, Schmidt G, Rudland PS: Development of hyperplasias, preneoplasias, and mammary tumors in MMTV-c-erbB-2 and MMTV-TGFalpha transgenic rats. Am J Pathol 155: 303–314, 1999
Singh M, McGinley JN, Thompson HJ: A comparison of the histopathology of premalignant and malignant mammary gland lesions induced in sexually immature rats with those occurring in the human. Lab Invest 80: 221–231, 2000
Medina D: Biological and molecular characteristics of the premalignant mouse mammary gland. Biochim Biophys Acta 1603: 1–9, 2002
Said TK, Medina D: Cell cyclins and cyclin-dependent kinase activities in mouse mammary tumor development. Carcinogenesis 16: 823–830, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hamaguchi, T., Matsuoka, Y., Kawaguchi, H. et al. Terminal Endbuds and Acini as the Respective Major Targets for Chemical and Sporadic Carcinogenesis in the Mammary Glands of Human c-Ha-ras Protooncogene Transgenic Rats. Breast Cancer Res Treat 83, 43–56 (2004). https://doi.org/10.1023/B:BREA.0000010698.09512.d2
Issue Date:
DOI: https://doi.org/10.1023/B:BREA.0000010698.09512.d2