Abstract
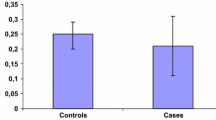
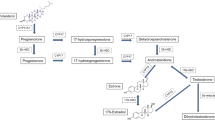
In order to investigate the influence of estrogenmetabolism on human breast cancer, estradiol 2- and16α-hydroxylase (2- and 16α-OHase) activities were determined inthe microsomal fractions of cancer tissues by usingreverse phase HPLC. 2-OHase activity was detected inmost cancer tissues and noncancerous tissues, but theactivity was significantly lower in cancer tissues thanin the paired noncancerous tissues (0.01 < p< 0.02). Interestingly the patients without lymph nodemetastasis had significantly higher 2-OHase activity in cancertissues than those with lymph node metastasis (0.02< p < 0.05). No correlation was observedbetween ER status and 2-OHase activity in cancertissues. On the other hand, 16α-OHase activity wasdetected only in one third of the breastcancer tissues examined. The activity was not significantlydifferent from that in noncancerous tissues, although itwas relatively higher in ER-positive cancer tissues whencompared with that in ER-negative ones (0.05 <p < 0.1). Estrone sulfatase activity measured simultaneouslyin the cytosol fractions of some specimens wasmuch higher in cancer tissues than in noncanceroustissues (0.02 < p < 0.05). We found,however, no correlation between estrone sulfatase activity andestradiol hydroxylase activity. Taken together, our results suggestthat the increase in 2-OHase activity prevents theproliferation of breast cancer and that estradiol metabolismis regulated independently of the local biosynthesis ofestrogen.
Similar content being viewed by others
References
Fishman J: Endocrine participation in the etiology of breast cancer. In: Harris JR, Hellman S, Henderson IC, Kinne DW (eds) Breast Diseases. JB Lippincott Company, Philadelphia, 1987, pp 103–109
Schneider J, Huh MM, Bradlow HL, Fishman J: Antiestrogen action of 2-hydroxyestrone on MCF-7 human breast cancer cells. J Biol Chem 259: 4840–4845, 1984
Swaneck GE, Fishman J: Covalent binding of the endogenous estrogen 16α-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization. Proc Natl Acad Sci 85: 7831–7835, 1988
Seegers JC, Aveling M, Aswegwn CH, Cross M, Koch F, Joubert WS: The cytotoxic effects of estradiol-17β, catecholestradiols and methoxyestradiols on dividing MCF-7 and Hela cells. J Steroid Biochem 32: 797–809, 1989
Lottering M, Haag M, Seegers JC: Effects of 17β-estradiol metabolites on cell cycle events in MCF-7 cells. Cancer Res 52: 5926–5932, 1992
Anderson KE, Kappas A, Conney AH, Bradlow HL, Fishman J: The influence of dietary protein and carbohydrate on the principal oxidative biotransformations of estradiol in normal subjects. J Clin Endocrinol Metab 59: 103–107, 1984
Michnovicz JJ, Bradlow HL: Induction of estradiol metabolism by dietary indole-3-carbinol in humans. J Natl Cancer Inst 82: 947–949, 1990
Michnovicz JJ, Bradlow HL: Altered estrogen metabolism and excretion in humans following consumption of indole-3-carbinol. Nutr Cancer 16: 59–66, 1991
Jellinck PH, Michnovicz JJ, Bradlow HL: Influence of indole-3-carbinol on the hepatic microsomal formation of catechol estrogens. Steroids 56: 446–450, 1991
Schneider J, Kinne D, Fracchia A, Pierce V, Anderson KE, Bradlow HL, Fishman J: Abnormal oxidation metabolism of estradiol in women with breast cancer. Proc Natl Acad Sci 79: 3047–3051, 1982
Bradlow HL, Hershcopf RJ, Martucci CP, Fishman J: Estradiol 16α-hydroxylation in the mouse correlates with mammary tumor incidence and presence of murine mammary tumor virus: a possible model for the hormonal etiology of breast cancer in humans. Proc Natl Acad Sci 82: 6295–6299, 1985
Bradlow HL, Hershcopf R, Martucci CP, Fishman J: 16α-hydroxylation of estradiol: a possible risk marker for breast cancer. Ann N.Y. Acad Sci 464: 138–151, 1986
Telang NT, Suto A, Wong GY, Osborne MP, Bradlow HL: Induction by estrogen metabolite 16α-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells. J Natl Cancer Inst 84: 634–638, 1992
Suto A, Bradlow HL, Wong GYC, Osborne MP, Telang NT: Experimental down-regulation of intermediate biomarkers of carcinogenesis in mouse mammary epithelial cells. Breast Cancer Res Treat 27: 193–202, 1993
Osborne MP, Bradlow HL, Wong YC, Telang NT: Upregulation of estradiol C16α-hydroxylation in human breast tissue: a potential biomarker of breast cancer risk. J Natl Cancer Inst 85: 1917–1920, 1993
Hoffman AR, Paul SM, Axelrod J: Catecholestrogen synthesis and metabolism by human breast tumors. Cancer Res 39: 4584–4587, 1979
Abul-hajj YJ, Thijssen JHH, Blankenstein MA: Metabolism of estradiol by human breast cancer. Eur J Cancer Clin Oncol 24: 1171–1178, 1988
Niwa T, Bradlow HL, Fishman J, Swaneck G: Induction and inhibition of estradiol hydroxylase activities in MCF-7 human breast cancer cells in culture. Steroids 55: 297–302, 1990
Lowry OH, Rosenbrogh NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–276, 1951
Utsumi T: Local production of estrogen via aromatase and estrone sulfatase in breast cancer tissues. (in Japanese) Nippon-Geka-Gakkai Zasshi 90: 920–927, 1989
Lemon HM, Heidel JW, Rodriguez-Sierra JF: Increased catecholestrogen metabolism as a risk factor for nonfamilial breast cancer. Cancer 69: 457–465, 1992
Roy D, Floyd RA, Liehr JG: Elevated 8-hydroxy-deoxyguanosine levels in DNA of diethylstilbesterol-treated Syrian hamsters: covalent DNA damage by free radicals generated by redox cycling of diethylstilbesterol. Cancer Res 51: 3882–3885, 1991
Santner SJ, Feil PD, Santen RJ: In situ estrogen production via the estrone sulfatase pathway in breast tumors: relative importance versus the aromatase pathway. J Clin Endocrinol 59: 29–33, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Imoto, S., Mitani, F., Enomoto, K. et al. Influence of estrogen metabolism on proliferation of human breast cancer. Breast Cancer Res Treat 42, 57–64 (1997). https://doi.org/10.1023/B:BREA.0000010495.08233.09
Issue Date:
DOI: https://doi.org/10.1023/B:BREA.0000010495.08233.09