Abstract
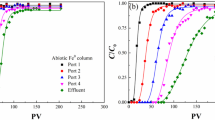
Dissolved hydrogen (H2) concentrations have been shown to correlate with specific terminal electron accepting processes (TEAPs) in aquifers. The research presented herein examined the effect of iron bioavailability on H2 concentrations during iron reduction in flow-through column experiments filled with soil obtained from the uncontaminated background area of the Field Research Center (FRC), Oak Ridge, TN and amended with acetate as the electron donor. The first column experiment measured H2 concentrations over 500 days of column operation that fluctuated within a substantial range around an average of 3.9 nM. Iron reduction was determined to be the dominant electron accepting process. AQDS (9,10-anthraquinone-2,6-disulfonic acid) was then used to determine if H2 concentrations during iron reduction were related to iron bioavailability. For this purpose, a 100-day flow-through column experiment was conducted that compared the effect of AQDS on iron reduction and subsequent H2 concentrations using two columns in parallel. Both columns were packed with FRC soil and inoculated with Geobacter sulfurreducens but only one was supplied with AQDS. The addition of AQDS increased the rate of iron reduction in the flow-through column and slightly decreased the steady-state H2 concentrations from an average of 4.0 nM for the column without AQDS to 2.0 nM for the column with AQDS. The results of this study therefore show that H2 can be used as an indicator to monitor rate and bioavailability changes during microbial iron reduction.
Similar content being viewed by others
References
Anderson RT, Rooney-Varga IN, Gaw CV & Lovley DR (1998) Anaerobic benzene oxidation in the Fe(III) reduction zone of petroleum contaminated aquifers. Environ. Sci. Technol. 32: 1222–1229
Anderson RT, Vrionis HA, Ortiz-Bernad I, Resch CT, Long PE, Dayvault R, Karp K, Marutzky S, Metzler DR, Peacock A, White DC, Lowe M & Lovley DR (2003) Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69: 5884–5891
Brown DG, Komlos J & Jaffe PR (in review) Simultaneous utilization of acetate and hydrogen by Geobacter sulfurreducens and the implications for use of hydrogen as an indicator of redox conditions
Caccavo F, Lonergan DJ, Lovley DR, Davis M, Stolz JF & McInerney MJ (1994) Geobacter sulfurreducens sp. nov., a hydrogen-and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60: 3752–3759
Chapelle FH, McMahon PB, Dubrovsky NM, Fujii RF, Oaksford ET & Vroblesky DA (1995) Deducing the distribution of terminal electron-accepting processes in hydrologically diverse groundwater systems. Water Resour. Res. 31: 359–371
Chapelle FH, Haack SK, Adriaens P, Henry MA & Bradley PM (1996) Comparison of E h and H2 measurements for delineating redox processes in a contaminated aquifer. Environ. Sci. Technol. 30: 3565–3569
Chapelle FH, Vroblesky DA, Woodward JC & Lovley DR (1997) Practical considerations for measuring hydrogen concentrations in groundwater. Environ. Sci. Technol. 31: 2873–2877
Chapelle FH, Bradley PM, Lovley DR, O'Neill K & Landmeyer JE (2002) Rapid evolution of redox processes in a petroleum hydrocarbon-contaminated aquifer. Ground Water 40: 353–360
Hacherl EL, Kosson DS, Young LY & Cowan RM (2001) Measurement of iron(III) bioavailability in pure iron oxide minerals and soils using anthraquinone-2,6-disulfonate oxidation. Environ. Sci. Technol. 35: 4886–4893
Hoehler TM, Alperin MJ, Albert DB & Martens CS (1998) Thermodynamic control on hydrogen concentrations in anoxic sediments. Geochim. Cosmochim. Acta 62: 1745–1756
Istok JD, Senko JM, Krumholz RL, Watson D, Bogle MA, Peacock A, Chang YJ & White DC (2004) In situ bioreduction of technetium and uranium in a nitrate-contaminated aquifer. Environ. Sci. Technol. 38: 468–475
Lovley DR & Phillips EJP (1987a) Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 53: 1536–1540
Lovley DR & Phillips EJP (1987b) Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl. Environ. Microbiol. 53: 2636–2641
Lovley DR & Goodwin S (1988) Hydrogen concentrations as an indicator of the predominant terminal electron accepting 325 reactions in aquatic sediments. Geochim. Cosmochim. Acta 52: 2993–3003
Lovley DR & Phillips EJP (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54: 1472–1480
Lovley DR, Chapelle FH & Woodward JC (1994) Use of dissolved H2 concentrations to determine distribution of microbially catalyzed reactions in anoxic groundwater. Environ. Sci. Technol. 28: 1205–1210
Lovley DR, Coates JD, Blunt-Harris EL, Phillips EJP & Woodward JC (1996) Humic substances as electron acceptors for microbial respiration. Nature 382: 445–448
Nevin KP & Lovley DR (2000) Potential for nonenzymatic reduction of Fe(III) via electron shuttling in subsurface sediments. Environ. Sci. Technol. 34: 2472–2478
Vroblesky DA, Bradley PM & Chapelle FH (1996) Influence of electron donor on the minimum sulfate concentration required for sulfate reduction in a petroleum hydrocarboncontaminated aquifer. Environ. Sci. Technol. 30: 1377–1381
Watson IA, Oswald SE, Mayer KU, Wu Y & Banwart SA (2003) Modeling kinetic processes controlling hydrogen and acetate concentrations in an aquifer-derived microcosm. Environ. Sci. Technol. 37: 3910–3919
Wilhelm E, Battino R & Wilcock RJ (1977) Low-pressure solubility of gases in liquid water. Chem. Rev. 77: 219–262
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Komlos, J., Jaffé, P.R. Effect of Iron Bioavailability on Dissolved Hydrogen Concentrations During Microbial Iron Reduction. Biodegradation 15, 315–325 (2004). https://doi.org/10.1023/B:BIOD.0000042187.31072.60
Issue Date:
DOI: https://doi.org/10.1023/B:BIOD.0000042187.31072.60