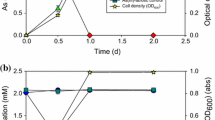
Abstract
Due to the recent enactment of a stricter drinking water standard for arsenate, large quantities of arsenate-laden drinking water residuals will be disposed in municipal landfills. The objective of this study was to determine the role of methanogenic consortia on the conversion of arsenate. Methanogenic conditions commonly occur in mature municipal solid waste landfills. The results indicate the rapid and facile reduction of arsenate to arsenite in methanogenic sludge. Endogenous substrates in the sludge were sufficient to support the reductive biotransformation. However the rates of arsenate reduction were stimulated by the addition of exogenous electron donating substrates, such as H2, lactate or a mixture of volatile fatty acids. A selective methanogenic inhibitor stimulated arsenate reduction in microcosms supplied with H2, suggesting that methanogens competed with arsenate reducers for the electron donor. Rates of arsenate reduction increased with arsenate concentration up to 2 mM, higher concentrations were inhibitory. The electron shuttle, anthraquinone-2,6-disulfonate, used as a model of humic quinone moieties, was shown to significantly increase rates of arsenate reduction at substoichiometric concentrations. The presence of sulfur compounds, sulfate and sulfide, did not affect the rate of arsenate transformation but lowered the yield of soluble arsenite, due to the precipitation of arsenite with sulfides. The results taken as a whole suggest that arsenate disposed into anaerobic environments may readily be converted to arsenite increasing the mobility of arsenic. The extent of the increased mobility will depend on the concentration of sulfides generated from sulfate reduction.
Similar content being viewed by others
References
Amy G, Edwards M, Brandhuber P, McNeill L, Benjamin M, Vagliasindi F, Carlson K & Chwirka J (2000) Arsenic treatability options and evaluation of residuals management issues. American Water Works Association Research Foundation, Denver
APHA (1998) Standard mthods for the examination of water and wastewater. American Public Health Association, Washington, DC
Blum JS, Bindi AB, Buzzelli J, Stolz JF & Oremland RS (1998) Bacillus arsenicoselenatis, sp nov, and Bacillus selenitireducens, sp nov: two haloalkaliphiles from Mono Lake, California that respire oxyanions of selenium and arsenic. Arch. Microbiol. 171: 19–30
Carbonell-Barrachina AA, Jugsujinda A, Burlo F, Delaune RD & Patrick WH (2000) Arsenic chemistry in municipal sewage sludge as affected by redox potential and pH. Wat. Res. 34: 216–224
Cervantes FJ, de Bok FAM, Tuan DD, Stams AJM, Lettinga G & Field JA (2002) Reduction of humic substances by halorespiring, sulphate-reducing and methanogenic microorganisms. Environ. Microbiol. 4: 51–57
Cervantes FJ, van der Velde S, Lettinga G & Field JA (2000) Competition between methanogenesis and quinone respiration for ecologically important substrates in anaerobic consortia. FEMS Microbiol. Ecol. 34: 161–171
Christensen TH, Kjeldsen P, Bjerg PL, Jensen DL, Christensen JB, Baun A, Albrechtsen HJ & Heron C (2001) Biogeochemistry of landfill leachate plumes. Appl. Geochem. 16: 659–718
Dowdle PR, Laverman AM & Oremland RS (1996) Bacterial dissimilatory reduction of arsenic(V) to arsenic(III) in anoxic sediments. Appl. Environ. Microbiol. 62: 1664–1669
Fernandez A, Hashsham SA, Dollhopf SL, Raskin L, Glagoleva O., Dazzo FB, Hickey R, Criddle C & Tiedje JM (2000) Flexible community structure correlates with stable community function in methanogenic bioreactor communities perturbed by glucose. Appl. Environ. Microbiol. 66: 4058–4067
Fischer K, Chodura A, Kotalik J, Bieniek D & Kettrup A (1997) Analysis of aliphatic carboxylic acids and amino acids in effluents of landfills, composting plants and fermentation plants by ion-exclusion and ion-exchange chromatography. J. Chromatogr. A 770: 229–241
Forsberg CW (1978) Some effects of arsenic on the rumen micro-flora: an in vitro study. Can. J. Microbiol. 24: 36–44
Frigon JC, Bisaillon JG, Paquette G & Beaudet R (1997) Anaerobic treatment of a municipal landfill leachate. Can. J. Microbiol. 43: 937–944
Gladysheva TB, Oden KL & Rosen BP (1994) Properties of the rsenate reductase of plasmid R773. Biochemistry 33: 7288–7293
Godon J-J, Zumstein E, Dabert P, Habouzit F & Moletta R (1997) Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl. Environ. Microbiol. 63: 2802–2813
Gong ZL, Lu XF, Cullen WR & Le XC (2001) Unstable trivalent arsenic metabolites, monomethylarsonous acid and dimethylarsinous acid. J. Anal. At. Spectrom. 16: 1409–1413
Harrington JM, Fendorf SE & Rosenzweig RF (1998) Biotic generation of arsenic(III) in metal(loid)-contaminated freshwater lake sediments. Environ. Sci. Technol. 32: 2425–2430
Helz GR, Tossell JA, Charnock JM, Pattrick RAD, Vaughan DJ & Garner CD (1995) Oligomerization in As(III) sulfide solutions - Theoretical constraints and spectroscopic evidence. Geochim. Cosmochim. Acta 59: 4591–4604
Herbel MJ, Blum JS, Hoeft SE, Cohen SM, Arnold LL, Lisak J, Stolz JF & Oremland RS (2002) Dissimilatory arsenate reductase activity and arsenate-respiring bacteria in bovine rumen fluid, hamster feces, and the termite hindgut. FEMS Microbiol. Ecol. 41: 59–67
Hoeft SE, Lucas F, Hollibaugh JT & Oremland RS (2002) Characterization of microbial arsenate reduction in the anoxic bottom waters of Mono Lake, California. Geomicrobiol. J. 19: 23–40
Hristova KR, Mau M, Zheng D, Aminov RI, Mackie RI, Gaskins HR & Raskin L (2000) Desulfotomaculum genus-and subgenus-specific 16S rRNA hybridization probes for environmental studies. Environ. Microbiol. 2: 143–159
Huber R, Sacher M, Vollmann A, Huber H & Rose D (2000) Respiration of arsenate and selenate by hyperthermophilic archaea. Syst. Appl. Microbiol. 23: 305–314
Imachi H, Sekiguchi Y, Kamagata Y, Ohashi A & Harada H (2000) Cultivation and in situ detection of a thermophilic bacterium capable of oxidizing propionate in syntrophic association with hydrogenotrophic methanogens in a thermophilic methanogenic granular sludge. Appl. Environ. Microbiol. 66: 3608–3615
Inskeep WP, McDermott TR & Fendorf S (2002) Arsenic (V)/(III) cycling in soils and natural waters: chemical and microbiological processes. In: Frankenberger WT (Ed) Environmental Chemistry of Arsenic (pp 183–215). Marcel Dekker, Inc., New York
Jones CA, Langner HW, Anderson K, McDermott TR & Inskeep WP (2000) Rates of microbially mediated arsenate reduction and solubilization. Soil Sci. Soc. Am. J. 64: 600–608
Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A & Christensen TH (2002) Present and long-term composition of MSW landfill leachate: A review. Crit. Rev. Environ. Sci. Technol. 32: 297–336
Krafft T & Macy JM (1998) Purification and characterization of the respiratory arsenate reductase of Chrysiogenes arsenatis. Eur. J. Biochem. 255: 647–653
Kuai L, Nair AA & Polz MF (2001) Rapid and simple method for the most-probable-number estimation of arsenic-reducing bacteria. Appl. Environ. Microbiol. 67: 3168–3173
Laverman AM, Switzer Blum J, Schaefer JK, Phillips EJP, Lovley DR & Oremland RS (1995) Growth of strain SES-3 with arsenate and other diverse electron acceptors. Appl. Environ. Microbiol. 61: 3556–3561
Lin TF & Wu JK (2001) Adsorption of arsenite and arsenate within activated alumina grains: equilibrium and kinetics. Wat. Res. 35: 2049–2057
Lovley DR, Coates JD, BluntHarris EL, Phillips EJP & Woodward JC (1996) Humic substances as electron acceptors for microbial respiration. Nature 382: 445–448
Lovley DR, Fraga JL, Blunt-Harris EL, Hayes LA, Phillips EJP & Coates JD (1998) Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim. Hydrobiol. 26: 152–157
Lovley DR, Fraga JL, Coates JD & Blunt-Harris EL (1999) Humics as an electron donor for anaerobic respiration. Environ. Microbiol. 1: 89–98
Ludvigsen L, Albrechtsen HJ, Ringelberg DB, Ekelund F & Christensen TH (1999) Distribution and composition of microbial populations in landfill leachate contaminated aquifer (Grindsted, Denmark). Microb. Ecol. 37: 197–207
Macy JM, Nunan K, Hagen KD, Dixon DR, Harbour PJ, Cahill M & Sly LI (1996) Chrysiogenes arsenatis gen. nov., sp. nov., a new arsenate-respiring bacterium isolated from gold mine wastewater. Int. J. Syst. Bacteriol 46: 1153–1157
Macy JM, Santini JM, Pauling BV, O'Neill AH & Sly LI (2000) Two new arsenate/sulfate-reducing bacteria: mechanisms of arsenate reduction. Arch. Microbiol. 173: 49–57
McCreadie H & Blowes DW (2000) Influence of reduction reactions and solid phase composition on porewater concentrations of arsenic. Environ. Sci. Technol. 34: 3159–3166
Meng XG, Korfiatis GP, Jing CY & Christodoulatos C (2001) Redox transformations of arsenic and iron in water treatment sludge during aging and TCLP extraction. Environ. Sci. Technol. 35: 3476–3481
Michalke K, Wickenheiser EB, Mehring M, Hirner AV & Hensel R (2000) Production of volatile derivatives of metal(loid)s by microflora involved in anaerobic digestion of sewage sludge. Appl. Environ. Microbiol. 66: 2791–2796
Mukhopadhyay R, Rosen BP, Pung LT & Silver S (2002) Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol. Rev. 26: 311–325
Newman DK, Ahmann D & Morel FMM (1998) A brief review of microbial arsenate respiration. Geomicrobiol. J. 15: 255–268
Newman DK, Beveridge TJ & Morel FMM (1997a) Precipitation of arsenic trisulfide by Desulfotomaculum auripigmentum. Appl. Environ. Microbiol. 63: 2022–2028
Newman DK, Kennedy EK, Coates JD, Ahmann D, Ellis DJ, Lovley DR & Morel FMM (1997b) Dissimilatory arsenate and sulfate reduction in Desulfotomaculum auripigmentum sp. nov. Arch. Microbiol. 168: 380–388
Niggemyer A, Spring S, Stackebrandt E & Rosenzweig RF (2001) Isolation and characterization of a novel As(V)-reducing bacterium: Implications for arsenic mobilization and the genus Desulfitobacterium. Appl. Environ. Microbiol. 67: 5568–5580
Oremland RS, Dowdle PR, Hoeft S, Sharp JO, Schaefer JK, Miller LG, Blum JS, Smith RL, Bloom NS & Wallschlaeger D (2000) Bacterial dissimilatory reduction of arsenate and sulfate in meromictic Mono Lake, California. Geochim. Cosmochim. Acta 64: 3073–3084
Oremland RS & Stolz JF (2003) The ecology of arsenic. Science 300: 939–944
Raskin L, Rittman BE & Stahl DA (1996) Competition and coexistence of sulfate-reducing and methanogenic populations in anaerobic biofilms. Appl. Environ. Microbiol. 62: 3847–3857
Rittle KA, Drever JI & Colberg PJS (1995) Precipitation of arsenic during bacterial sulfate reduction. Geomicrobiol. J. 13: 1–11
Rochette EA, Bostick BC, Li GC & Fendorf S (2000) Kinetics of arsenate reduction by dissolved sulfide. Environ. Sci. Technol. 34: 4714–4720
Rosen BP (2002) Biochemistry of arsenic detoxification. FEBS Letters 529: 86–92
Saltikov CW, Cifuentes A, Venkateswaran K & Newman DK (2003) The ars detoxification system is advantageous but not required for As(V) respiration by the genetically tractable Shewanella species strain ANA-3. Appl. Environ. Microbiol. 69: 2800–2809
Saltikov CW & Newman DK (2003) Genetic identification of a respiratory arsenate reductase. Proc. Nat. Acad. Sci. USA 100: 10983–10988
Scholten JCM, Conrad R & Stams AJM (2000) Effect of 2-bromoethane sulfonate, molybdate and chloroform on acetate consumption by methanogenic and sulfate-reducing populations in freshwater sediment. FEMS Microbiol. Ecol. 32: 35–42
Scott DT, McKnight DM, Blunt-Harris EL, Kolesar SE & Lovley DR (1998) Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 32: 2984–2989
Sekiguchi Y, Y. Kamagata, K. Syutsubo, A. Ohashi, H. Harada & Nakamura K (1998) Phylogenetic diversity of mesophilic and thermophilic granular sludges determined by 16S rRNA gene analysis. Microbiology 144: 2655–2665
Stolz JF & Oremland RS (1999) Bacterial respiration of arsenic and selenium. FEMS Microbiol. Rev. 23: 615–627
Trüper HGaS, H. G. (1964) Sulphur metabolism in thiorhodaceae: Quantitative measurements on growing cells of Chromatium okenii. Antonie Leeuwen. 30: 225–238
US-EPA (2001) National primary drinking water regulations: arsenic and clarifications to compliance and new source contaminants monitoring. Federal Register 66: 6976–7066
Van Dyke MI & McCarthy AJ (2002) Molecular biological detection and characterization of Clostridium populations in municipal landfill sites. Appl. Environ. Microbiol. 68: 2049–2053
Van Lier JB, Grolle KCF, M. SAJ, Conway de Macario E & Lettinga G (1992) Start-up of a thermophilic Upflow Anaerobic Sludge Bed (UASB) reactor with mesophilic granular sludge. Appl. Microbiol. Biotechnol. 37: 130–135
Vieira AMS, Bergamasco R, Gimenes ML, Nakamura CV & Dias BP (2001) Microbial populations of an upflow anaerobic sludge blanket reactor treating wastewater from a gelatin industry. Environ. Technol. 22: 1477–1485
Wallace W, Ward T, Breen A & Attaway H (1996) Identification of an anaerobic bacterium which reduces perchlorate and chlorate as Wolinella succinogenes. J. Ind. Microbioll 16: 68–72
Webster JG (1990) The Solubility of As2S3 and Speciation of as in Dilute and Sulfide-Bearing Fluids at 25-Degrees-C and 90-Degrees-C. Geochim. Cosmochim. Acta 54: 1009–1017
Wickenheiser EB, Michalke K, Drescher C, Hirner AV & Hensel R (1998) Development and application of liquid and gaschromatographic speciation techniques with element specific (ICP-MS) detection to the study of anaerobic arsenic metabolism. Fresenius J. Anal. Chem. 362: 498–501
Zobrist J, Dowdle PR, Davis JA & Oremland RS (2000) Mobilization of arsenite by dissimilatory reduction of adsorbed arsenate. Environ. Sci. Technol. 34: 4747–4753
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Field, J.A., Sierra-Alvarez, R., Cortinas, I. et al. Facile reduction of arsenate in methanogenic sludge. Biodegradation 15, 185–196 (2004). https://doi.org/10.1023/B:BIOD.0000026697.10029.b2
Issue Date:
DOI: https://doi.org/10.1023/B:BIOD.0000026697.10029.b2