Abstract
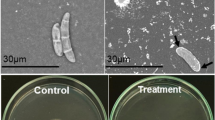
Endogenous chitinase plays a positive role in the pathogenicity of Bacillus thuringiensis to insect pests. The chitinase gene was cloned from B. thuringiensis serovar alesti strain HD-16, and the deduced 676 amino acid sequence showed a high degree of similarity with other Bacillus chitinases. Additionally, the deduced amino acid sequence showed that the protein contained an amino terminus signal peptide and consisted of a catalytic domain, a fibronectin type III domain and a chitin-binding domain. All three domains showed conserved sequences when compared to other bacterial chitinase or cellulase sequences.
Similar content being viewed by others
References
Arora N, Ahmad T, Rajagopal R, Bhatnagar RK (2003) A constitutively expressed 36 kDa exochitinase from Bacillus thuringiensis HD-1. Biochem. Biophys. Res. Commun. 307: 620-625.
Barboza-Corona JE, Nieto-Mazzocco E, Velazquez-Robledo R, Salcedo-Hernandez R, Bautista M, Jimenez B, Ibarra JE (2003) Cloning, sequencing, and expression of the chitinase gene chiA74 from Bacillus thuringiensis. Appl. Environ. Microbiol. 69: 1023-1029.
Marimoto K, Karita S, Kimura T, Sakka K, Ohmiya K (1997) Cloning, sequence, and expression of the gene encoding Clostridium paraputrificum chitinase ChiB and analysis of the functions of novel cadherin-like domains and a chitin-binding domain. J. Bacteriol. 179: 7306-7314.
Regev A, Keller M, Strizhov N, Sneh B, Prudovsky E, Chet I, Ginzberg I, Koncz-Kalman Z, Koncz C, Schell J, Zilberstein A (1996) Synergistic activity of a Bacillus thuringiensis δ-endotoxins and a bacterial endochitinase against Spodoptera littoralis larvae. Appl. Environ. Microbiol. 62: 3581-3586.
Ruoslahti E (1988) Fibronectin and its receptors. Annu. Rev. Biochem. 57: 375-413.
Sampson MN, Gooday GW(1998) Involvement of chitinases of Bacillus thuringiensis during pathogenesis in insects. Microbiology 144: 2189-2194.
Thamthiankul S, Suan-Ngay S, Tantimavanich S, Panbangred W (2001) Chitinase from Bacillus thuringiensis subsp. pakistani. Appl. Microbiol. Biotechnol. 56: 395-401.
Watanabe T, Kobori K, Miyashita K, Fujii T, Sakai M, Uchida M, Tamaka H (1993) Identification of glutamic acid 201 and aspartic 200 in chitinase A1 of Bacillus circulans WL-12 as essential residues for chitinase activity. J. Biol. Chem. 268: 18567-18572.
Watanabe T, Uchida M, Kabori K, Tanaka H (1994) Site-directed mutagenesis of the Asp-197 and Asp-202 in the chitinase A1 of Bacillus circulans WL-12. Biosci. Biotechnol. Biochem. 58: 2283-2285.
Wiwat C, Thaithanum S, Pantuwatana S, Bhumiratana A (2000) Toxicity of chitinase-producing Bacillus thuringiensis ssp. kurstaki HD-1 (G) toward Plutella xylostella. J. Invertebr. Pathol. 76: 270-277.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lin, Y., Xiong, G. Molecular cloning and sequence analysis of the chitinase gene from Bacillus thuringiensis serovar alesti . Biotechnology Letters 26, 635–639 (2004). https://doi.org/10.1023/B:BILE.0000023021.50213.ed
Issue Date:
DOI: https://doi.org/10.1023/B:BILE.0000023021.50213.ed