Abstract
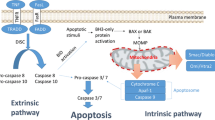
The Inhibitor of Apoptosis Protein family (IAP) functions as inhibitors of apoptotic pathways, both death receptor- and mitochondrial mediated. We detail the current body of knowledge for the IAP family with regard to their structure and function, their expression in normal and leukemic cells, and their prognostic importance in acute leukemia. Although there is some evidence that IAPs play an important role in the chemoresistance of leukemia cell lines, little is known about their influence on this phenomenon in acute leukemia cells of human origin. IAPs are also explored as a specific target for new antitumor strategies, including antisense oligonucleotides of XIAP (X-chromosome-linked IAP) or survivin and small molecules of polyphenylurea-based XIAP inhibitors.
Several proteins negatively regulate the function of the IAP family. One of those antagonists is Smac/DIABLO. Short peptides of Smac were found to enhanced apoptosis, induced by chemo- or immunotherapy, in the leukemic cells in vitro. Moreover, small-molecule agents, resembling Smac/DIABLO in function, were shown to potentiate cytotoxicity of chemotherapy in different malignancies.
IAPs, exhibiting downstream influence on both external and intrinsic pathways as well as on some caspase-independent mechanisms of apoptosis, are potentially attractive target for anti-tumor therapy, although their role in the pathology and prognosis of acute leukemia has to be further elucidated.
Similar content being viewed by others
References
Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994; 73: 2013–2026.
Lin CW, Manshouri T, Jilani I, et al. Proliferation and apoptosis in acute and chronic leukemias and myelodysplastic syndrome. Leukemia Res 2002; 26: 551–559.
Smolewski P, Darzynkiewicz Z, Robak T. Caspase-mediated cell death in hematological malignancies: Theoretical considerations, methods of assessment, and clinical implications. Leuk Lymphoma 2003; 44: 1089–1104.
Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Ann Rev Biochem 1999; 68: 383–424.
Nicholson DW. Caspase structure, proteolytic substrates and function during apoptotic cell death. Cell Death and Differ 1999; 6: 1028–1042.
Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 2001; 410: 549–554.
Jia L, Patwari Y, Kelsey SM, et al. Role of Smac in human leukaemic cell apoptosis and proliferation. Oncogene 2003; 22: 1589–1599.
Carson JP, Behnam M, Sutton JN, et al. Smac is required for cytochrome c-induced apoptosis in prostate cancer LNCaP cells. Cancer Res 2002; 62: 18–23.
Carter BZ, Kornblau SM, Tsao T, et al. Caspase-independent cell death in AML: Caspase inhibition in vitro with pan-caspase inhibitors or in vivo by XIAP or Survivin does not affect cell survival or prognosis. Blood 2003; 12: 4179–4186.
Tamm I, Kornblau SM, Segall H, et al. Expression and prognostic significance of IAP family genes in human cancers and myeloid leukemias. Clin Cancer Res 2000; 6: 1796–1803.
Adida C, Recher C, Raffoux E, et al. Expression and prognostic significance of survivin in de novo acute myeloid leukemia. Br J Haematol 2000; 111: 196–203.
Verhagen AM, Coulson EJ, Vaux DL. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol 2001; 2: Reviews 3009. 1–3009.10.
Takahashi R, Deveraux Q, Tamm I, et al. Asingle BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem 1998; 273: 7787–7790.
Zhivotovsky B, Orrenius S. Defects in the apoptotic machinery of cancer cells: Role in drug resistance. Sem Cancer Biol 2003; 13: 125–134.
Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: There is more to life than Bcl2. Oncogene 2003; 22: 8568–8580.
Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 2000; 288: 874–877.
Holley CL, Olson MR, Colo-Ramos DA, Kornbluth S. Reaper eliminates IAP proteins through stimulated IAP degradation and generalized translational inhibition. Nat Cell Biol 2002; 4: 439–444.
Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect Fas-induced cell death. Proc Natl Acad Sci USA 2001; 98: 8662–8667.
Hu S, Yang X. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J Biol Chem 2003; 278: 10055–10060.
Nakayama K, Kamihira S. Survivin an important determinant for prognosis in adult T-cell leukemia: A novel biomarker in practical hematooncology. Leuk Lymphoma 2002; 43: 2249–2255.
Chiou SK, Jones MK, Tarnawski AS. Survivin—an anti-apoptosis protein: Its biological roles and implications for cancer and beyond. Med Sci Monit 2003; 9: PI25–29.
O'Connor DS, Grossman D, Plescia J, et al. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA 2000; 97: 13103–13107.
Fukuda S, Mantel CR, Pelus LM. Survivin regulates hematopoietic progenitor cell proliferation through p21 WAF1 Cip1-dependent and-independent pathways. Blood 2004; 103: 120–127.
Beltrami E, Plescia J, Wilkinson JC, Duckett CS, Altieri DC. Acute ablation of survivin uncovers p53-dependent mitotic checkpoint functions and control of mitochondrial apoptosis. J Biol Chem 2004; 279: 2077–2084.
Suzuki A, Shiraki K. Tumor cell ‘dead or alive’: Caspase and survivin regulate cell death, cell cycle and cell survival. Histol Histopathol 2001; 16: 583–593.
Liu T, Brouha B, Grossman D. Rapid induction of mitochondrial events and caspase-independent apoptosis in Survivin-targeted melanoma cells. Oncogene 2004; 23: 39–48.
Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol 2001; 21: 3604–3608.
Hikita S, Hatano M, Inoue A, et al. Overexpression of TIAP/m-survivin in thymocytes enhances cell proliferation. Mol Immunol 2002; 39: 289–298.
Xing Z, Conway EM, Kang C, Winoto A. Essential role of survivin, an inhibitor of apoptosis protein, in T cell development, maturation, and homeostasis. J Exp Med 2004; 199: 69–80.
Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature 2002; 416: 345–347.
Hasegawa T, Suzuki K, Sakamoto C, et al. Expression of the inhibitor of apoptosis (IAP) family members in human neutrophils: Up-regulation of cIAP2 by granulocyte colony-stimulating factor and overexpression of cIAP2 in chronic neutrophilic leukemia. Blood 2003; 101: 1164–1171.
Li F, Ambrosini G, Chu EY, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998; 396: 580–584.
Fukuda, S., Pelus, L.M. Regulation of the inhibitor of apoptosis family member survivin in normal cord blood and bone marrow CD34 cells by hematopoietic growth factors: Implication of survivin expression in normal hematopoiesis. Blood 2001; 98: 2091–2100.
Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Estrov Z. Granulocyte-macrophage colony stimulating factor (GM-CSF) induces antiapoptotic and pro-apoptotic signals in acute myeloid leukemia. Blood 2003; 102: 630–637.
Carter BZ, Milella M, Altieri DC, Andreeff M. Cytokine-regulated expression of survivin in myeloid leukemia. Blood 2001; 97: 2784–2790.
Nakagawa Y, Yamaguchi S, Hasegawa M. Differential expression of survivin in bone marrow cells from patients with acute lymphocytic leukemia and chronic lympocytic leukemia. Leuk Res 2004; 28: 487–494.
Astier AL, Svoboda M, Hinds E, de Beaumont R, Munoz O, Freedman AS. Integrins regulate survival of pre-B-ALL cells through differential IAP and caspase-7 ubiquitination and degradation. Leukemia 2004; 18: 873–875.
Astier AL, Xu R, Svoboda M, et al. Temporal gene expression profile of human precursor B-leukemia cells induced by adhesion receptor: Identification of pathways regulating B-cell survival. Blood 2003; 101: 1118–1127.
Rajcan-Separovic E, Liston P, Lefebvre C, Korneluk RG. Assignment of human inhibitor of apoptosis protein (IAP) genes xiap, hiap-1, and hiap-2 to chromosomes Xq25 and 11q22-q23 by fluorescence in situ hybridization. Genomics 1996; 37: 404–406.
Neri LM, Borgatti P, Tazzari PL, et al. The phosphoinositide 3-kinase/AKT1 pathway involvement in drug and all-trans-retinoic acid resistance of leukemia cells. Mol Cancer Res 2003; 1: 234–246.
Chu Z-L, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Supression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA 1997; 94: 10057–10062.
Guo F, Nimmanapalli R, Paranawithana S, et al. Ectopic over-expression of second mitochondria-derived activator of caspases (Smac/DIABLO) or cotreatment with N-terminus of Smac/DIABLO peptide potentiates epothilone B derivative-( BMS 247550) and Apo-2L/TRAIL-induced apoptosis. Blood 2002; 99: 3419–3426.
Belka C, Schmid B, Marini P, et al. Sensitization of resistant lymphoma cells to irradiation-induced apoptosis by the death ligand TRAIL. Oncogene 2001; 20: 2190–2196.
Wen J, Ramadevi N, Nguyen D, Perkins C, Worthingogn E, Bhalla K. Antileukemic drugs increase death receptor 5 levels and enhance Apo-2/L-induced apoptosis of human acute leukemia cells. Blood 2000; 96: 3900–3906.
Hao XS, Hao JH, Liu FT, Newland AC, Jia L. Potential mechanisms of leukemia cell resistance to TRAIL-induced apoptosis. Apoptosis 2003; 8: 601–607.
Guo F, Sigua C, Tao J, et al. Cotreatment with histone deacetylase inhibitor LAQ824 enhances Apo-2L/tumor necrosis factor-related apoptosis inducing ligand-induced death inducing signaling complex activity and apoptosis of human acute leukemia cells. Cancer Res 2004; 64: 2580–2589.
Carter BZ, Milella M, Tsao T, et al. Regulation and targeting of antiapoptotic XIAP in acute myeloid leukemia. Leukemia 2003; 17: 2081–2089.
Schimmer AD, Welsh K, Pinilla C, et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell 2004; 5: 25–35.
Carter BZ, Wang RY, Schober WD, Milella M, Chism D, Andreeff M. Targeting Survivin expression induces cell proliferation defect and subsequent cell death involving mitochondrial pathway in myeloid leukemic cells. Cell Cycle 2003; 2: 488–493.
Tsuruma T, Hata F, Torigoe T. Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med 2004; 2: 19.
Zeis M, Siegel S, Wagner A, et al. Generation of cytotoxic responses in mice and human individuals against hematological malignancies using survivin-RNA-transfected dendritic cells. J Immunol 2003; 170: 5391–5397.
Srinivasula S, Hegde R, Saleh A, et al. Aconserved XIAP interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 2001; 410: 112–116.
Martins LM, Turk BE, Cowling V, et al. Binding specificity and regulation of the serine protease and PDZ domains of HtrA2/Omi. J Biol Chem 2003; 278: 49417–49427.
Li W, Srinivasula SM, Chai J, et al. Structural insights into the pro-apoptotic function of mitochondrial serine protease HtrA2/Omi. Nat Struct Biol 2002; 9: 436–441.
Jones JM, Datta P, Srinivasula SM, et al. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature 2003; 425: 721–727.
Maianski NA, Geissler J, Srinivasula SM, Alnemri ES, Roos D, Kuijpers TW. Functional characterization of mitochondria in neutrophils: A role restricted to apoptosis. Cell Death Differ 2004; 11: 143–153.
Jin S, Kalkum M, Overholtzer M, et al. CIAP1 and the serine protease HTRA2 are involved in a novel p53-dependent apoptosis pathway in mammals. Genes Dev 2003; 17: 359–367.
Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102: 33–42.
Hegde R, Srinivasula SM, Zhang ZJ, et al. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J Biol Chem 2002; 277: 432–438.
Gray CW, Ward RV, Karran E, et al. Characterization of human HtrA2, a novel serine protease involved in the mammalian cellular stress response. Eur J Biochem 2000; 267: 5699–5710.
Verhagen AM, Silke J, Ekert PG, et al. HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J Biol Chem 2003; 277: 445–454.
Srinivasula SM, Gupta S, Datta P, et al. Inhibitor of apoptosis proteins are substrates for the mitochondrial serine protease Omi/HtrA2. J Biol Chem 2003; 278: 31469–31472.
Suzuki Y, Takahashi-Niki H, Akagi T, Hashikawa T, Takahashi R. Mitochondrial protease Omi/HtrA2 enhances caspase activation through multiple pathways. Cell Death Differ 2004; 11: 208–216.
Leaman DW, Chawla-Sarkars M, Vyas K, et al. Identification of X-linked inhibitor of apoptosis-associated factor-1 as an interferon-stimulated gene that augments TRAIL Apo2L-induced apoptosis. J Biol Chem 2002; 277: 28504–28511.
Byun DS, Cho K, Ryu BK, et al. Hypermethylation of XIAP-associated factor 1, a putative tumor suppressor gene from the 17p13.2 locus, in human gastric adenocarcinomas. Cancer Res 2003; 63: 7068–7075.
Fu J, Jin J, Arend LJ. Smac3, a novel Smac/DIABLO splicing variant, attenuates the stability and apoptosis-inhibiting activity of X-linked inhibitor of apoptosis protein. J Biol Chem 2003; 278: 52660–52672.
Roberts DL, Merrison W, MacFarlane M, Cohen GM. The inhibitor of apoptosis protein-binding domain of Smac is not essential for its proapototic activity. J Cell Biol 2001; 153: 221–228.
Yang QH, Du C. Smac/DIABLO selectively reduces the levels of cIAP1 and cIAP2 but not that of XIAP and Livin in HeLa cells. J Biol Chem 2004; in press.
Liston P, Fong WG, Kelly NL, et al. Identification of XAF1 as an antagonist of XIAP anti-Caspase activity. Nat Cell Biol 2001; 3: 128–133.
Fong WG, Liston P, Rajcan-Separovic E, St Jean M, Craig C, Korneluk RG. Expression and genetic analysis of XIAP-associated factor 1(XAF1) in cancer cell lines. Genomics 2000; 70: 113–122.
Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 2000; 406: 855–862.
Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL-or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med 2002; 8: 808–815.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wrzesień-Kuś, A., Smolewski, P., Sobczak-Pluta, A. et al. The inhibitor of apoptosis protein family and its antagonists in acute leukemias. Apoptosis 9, 705–715 (2004). https://doi.org/10.1023/B:APPT.0000045788.61012.b2
Issue Date:
DOI: https://doi.org/10.1023/B:APPT.0000045788.61012.b2