Abstract
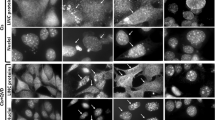
The mitochondrial localization of p53 is an important event in p53-dependent apoptosis. Some p53 mutants defective for transcription also facilitate apoptosis through changes of the mitochondria. Here, apoptosis of HeLa and CaSki cells (p53wt), C33A and HaCat cells (p53mt) and SaOs-2 cells (p53 deficient) was induced by 300 nM staurosporine. We showed that wild-type p53, as well as p53 mutants, were transiently located to the mitochondria with changes in the mitochondrial membrane potential (ΔΨm). However, in C33A cells harboring a p53 mutated on its DNA binding domain, ΔΨm collapse and Sub-G1 DNA content were reduced compared to p53wt cells, whereas no significant difference was observed in HaCat cells with a p53 mutated on UV hot spots. In addition, inhibition of the mitochondrial permeability transition pores by cyclosporine A significantly reduced the ΔΨm loss and the sub-G1 DNA content in p53 positive cells. These results indicate that ΔΨm collapse is an early and necessary event, which plays an important role in apoptosis of immortal mammalian cells.
Similar content being viewed by others
References
Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 2000; 7: 1166–1173.
Lutter M, Fang M, Luo X, Nishijima M, Xie X, Wang X. Cardiolipin provides specificity for targeting of tBid to mitochodria. Nat Cell Biol 2000; 2: 754–761.
Seo YW, Shin JN, Ko KH, et al. The molecular mechanism of Noxa-induced mitochondrial dysfunction in p53–mediated cell death. J Biol Chem 2003; 278: 48292–48299.
Mihara M, Erster S, Zaika A, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell 2003; 11: 577–590.
Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med 2000; 6: 513–519.
Shimizu S, Tsujimoto Y. Proapoptotic BH3–only Bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate voltage-dependent anion channel activity. Proc Natl Acad Sci USA 2000; 97: 577–582.
Kluck RM, Bossy-Wetzel E, Green Dr, Newmeyer DD. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 1997; 275: 1132–1136.
Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol 2000; 2: 156–162.
Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. Embo J 1998; 17: 37–49.
Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (Deltapsi(m)) in apoptosis; an update. Apoptosis 2003; 8: 115–128.
Daugas E, Susin SA, Zamzami N, et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. Faseb J 2000; 14: 729–739.
Daguas E, Nochy D, Ravagnan L, et al. Apoptosis-inducing factor (AIF): A ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett 2000; 476: 118–123.
Joseph B, Marchetti P, Formstecher P, Kroemer G, Lewensohn R, Zhivotovsky B. Mitochondrial dysfunction is an essential step for killing of non-small cell lung carcinomas resistant to conventional treatment. Oncogene 2002; 21: 65–77.
Susin SA, Zamzami N, Castedo M, et al. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med 1996; 184: 1331–1341.
He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: A new paradigm of pore structure and function? FEBS Lett 2002; 512: 1–7.
Griffiths EJ, Halestrap AP. Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase. Implications for the immunosuppressive and toxic effects of cyclosporin. Biochem J 1991; 274: 611–614.
Waring P, Beaver J. Cyclosporin A rescues thymocytes from apoptosis induced by very low concentrations of thapsigargin: Effects on mitochondrial function. Exp Cell Res 1996; 227: 264–276.
Mayer B, Oberbauer R. Mitochondrial regulation of apoptosis. New Physiol Sci 2003; 18: 89–94.
Tsujimoto Y. Bcl-2 family of proteins: Life-or-death switch in mitochondria. Biosci Rep 2002; 22: 47–58.
Shimizu S, Ide T, Yanagide T, Tsujimoto Y. Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J Biol Chem 2000; 275: 12321–12325.
Sugiyama T. Shimizu S, Matsuoka Y, Yoneda Y, Tsujimoto Y. Activation of mitochondrial voltage-dependent anion channel by apro-apoptotic BH3–only protein Bim. Oncogene 2002; 21: 4944–4956.
Antonsson B, Montessuit S, Lauper S, Eskes R, Martinous JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J 2000; 345 Pt 2: 271–278.
Vander Heiden MG, Thompson CB. Bcl-2 proteins: Regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol 1999; 1: E209–216.
Moll UM, Zaika A. Nuclear and mitochondrial apoptotic pathways of p53. FEBS Lett 2001; 493: 65–69.
Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem 2000; 275: 16202–16212.
Bernard B, Fest T, Prétet JL, Mougin C. Staurosporine-induced apoptosis of HPV positive and negative human cervical cancer cells from different points in the cell cycle. Cell Death Differ 2001; 8: 234–244.
Bernard B, Prétet JL, Charlot JF, Mougin C. Human papillomaviruses type 16+ and 18+ cervical carcinoma cells are sensitive to staurosporine-mediated apoptosis. Biol Cell 2003; 95: 17–26.
Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell 1999; 3: 159–167.
Scarlett JL, Sheard PW, Hughes G, Ledgerwood EC, Ku HH, Murphy MP. Changes in mitochondrial membrane potential during staurosporine-induced apoptosis in Jurkat cells. FEBS Lett 2000; 475: 267–272.
Xia T, Jiang C, Li L, Wu C, Chen Q, Liu SS. A study on permeability transition pore opening and cytochrome c release from mitochondria, induced by caspase-3 in vitro. FEBS Lett 2002; 510: 62–66.
Huang X, Zhai D, Huang Y. Dependence of permeability transition pore opening and cytochrome C release from mitochondria on mitochondria energetic status. Mol Cell Biochem 2001; 224: 1–7.
Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem 2001; 276: 12030–12034.
Ding WX, Shen HM, Ong CN. Calpain activation after mitochondrial permeability transition in microcystin-induced cell death in rat hepatocytes. Biochem Biophys Res Commun 2002; 291: 321–331.
Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabo I, Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem 1992; 267: 2934–2939.
Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem 1992; 267: 8834–8839.
Zamzami N, Marchetti P, Castedo M, et al. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med 1995; 181: 1661–1672.
Gottlieb RA, Granville DJ. Analyzing mitochondrial changes during apoptosis. Methods 2002; 26: 341–347.
Cohen GM. Caspases: The executioners of apoptosis. Biochem J 1997; 326: 1–16.
Heiskanen KM, Bhat MB, Wang HW, Ma J, Nieminen AL. Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells. J Biol Chem 1999; 274; 5655–5658.
Thornborrow EC, Patel S, Mastropietro AE, Schwartzfarb EM, Manfredi JJ. A conserved intronic response element mediates direct p53–dependent transcriptional activation of both the human and murine bax genes. Oncogene 2002; 21: 990–999.
Shimizu S, Konishi A, Kodama T, Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci USA 2000; 97: 3100–3105.
Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med 1997: 3: 614–620.
Crook T, Wrede D, Vousden KH. p53 point mutation in HPV negative human cervical carcinoma cell lines. Oncogene 1991; 6: 873–875.
Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem 1997; 174: 167–172.
Varbiro G, Veres B, Gallyas F, Jr., Sumegi B. Direct effect of Taxol on free radical formation and mitochondrial permeability transition. Free Radic Biol Med 2001; 31: 548–558.
Appaix F, Guerrero K, Rampal D, et al. Bax and heart mitochondria: Uncoupling and inhibition of respiration without permeability transition. Biochim Biophys Acta 2002; 1556: 155–167.
Regula KM, Kirshenbaum LA. p53 activates the mitochondrial death pathway and apoptosis of ventricular myocytes independent of de novo gene transcription. J Mol Cell Cardiol 2001; 33: 1435–1445.
Dumont P, Leu JI, Della Pietra AC, 3rd, George DL, Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet 2003; 33: 357–365.
Chipuk JE, Maurer U, Green DR, Schuler M. Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell 2003; 4: 371–381.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Charlot, J.F., Prétet, J.L., Haughey, C. et al. Mitochondrial translocation of p53 and mitochondrial membrane potential (ΔΨm) dissipation are early events in staurosporine-induced apoptosis of wild type and mutated p53 epithelial cells. Apoptosis 9, 333–343 (2004). https://doi.org/10.1023/B:APPT.0000025810.58981.4c
Issue Date:
DOI: https://doi.org/10.1023/B:APPT.0000025810.58981.4c