Abstract
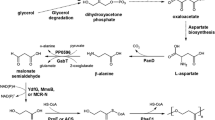
Heterogenous expression of (R)-3-hydroxydecanoyl-ACP:CoA transacylase gene (phaG) isolated from Pseudomonas putida in Escherichia coli HB101 led to the extracellular production of 3-hydroxydecanoic acid (3HD) in a growth medium consisting of carbon source non-related to 3HD structure, while no 3HD was detected in the growth media inoculated with wild type E. coli HB101 and recombinant E. coli HB101 harboring vector pBluescript SK− only. 3HD production by E. coli HB101 (pLZZGPp) harboring phaG from fructose was 587 mg/l, approximately three times that from cultivation in glucose under same culture conditions. 3HD production was affected by timing of fructose addition and fructose concentration in the culture. As an inhibitor of fatty acid de novo synthesis, the presence of triclosan in the culture could increase 3HD production by E. coli HB101 (pLZZGPp) by about 20–40%. The results further confirmed that (R)-3-hydroxydecanoyl-ACP:CoA transacylase (PhaG) provides 3HD precursors for medium-chain-length polyhydroxyalkanoate synthesis. At the same time, this phenomenon showed that recombinant organisms can be used for production of certain fine chemicals such as hydroxyalkanoic acids.
Similar content being viewed by others
References
Anderson A.J. and Dawes E.A. 1990. Occurrence, metabolism, metabolic role and industrial uses of bacterial polyhydroxy-alkanoates. Microbiol. Rev. 54: 450-472.
Anderson K.B. and von Meyenburg K. 1980. Are growth rates of Escherichia coli in batch cultures limited by respiration? J. Bacteriol. 144: 114-123.
Barnes E.M. Jr., Wakil S.J. and Swindell A.C. 1970. Purification and properties of a palmityl thioesterase II from Escherichia coli. J. Biol. Chem. 245: 3122-3128.
Byrom D. 1992. Production of poly-ß-hydroxybutyrate: poly-ß-hydroxyvalerate copolymers. 103: 247-250.
Chang D.E., Shin S., Rhee J.S. and Pan J.G. 1999. Acetate metabolism in pta mutant of Escherichia coli W3110: importance of maintaining acetyl coenzyme. A flux for growth and survival. J. Bacteriol. 181: 6656-6663.
Chen G.Q., Wu Q., Xi J., Yu H.P. and Chan A. 2000. Microbial production of biopolyesters-polyhydroxyalkanoates. Prog. Nat. Sci. 10: 843-850.
Chen G.Q., Zhang G., Park S.J. and Lee S.Y. 2001. Industrial production of poly(hydroxybutyrate-co-hydroxyhexanoate). Appl. Microbiol. Biotechnol. 57: 50-55.
DiRusso C.C., Black P.N. and Weimar J.D. 1999. Molecular inroads into the regulation and metabolism of fatty acids, lessons from bacteria. Prog. Lipid Res. 38: 129-197.
Fiedler S., Steinbüchel A. and Rehm B.H.A. 2000. PhaG-mediated synthesis of poly(3-hydroxyalkanoates) consisting of medium-chain-length constituents from nonrelated carbon sources in recombinant Pseudomonas fragi. Appl. Environ. Microbiol. 66: 2117-2124.
Fukui T., Shiomi N. and Doi Y. 1998. Expression and characterization of (R)-specific enoyl coenzyme A hydratase involved in polyhydroxyalkanoate biosynthesis by Aeromonas caviae. J. Bacteriol. 180: 667-673.
Hang X., Zhang G., Wang G., Zhao X. and Chen G.Q. 2002. PCR cloning of polyhydroxyalkanoate biosynthesis genes from Burkholderia caryphylli and their functional expression in recombinant Escherichia coli. FEMS Microbiol. Lett. (in press)
He W., Tian W., Zhang G., Chen G.Q. and Zhang Z. 1998. Production of novel polyhydroxyalkanoates by Pseudomonas stutzeri 1317 from glucose and soybean oil. FEMS Microbiol. Lett. 169: 45-49.
Heath R.J., Yu Y.T., Shapiro M.A., Olson E. and Rock C.O. 1998. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 273: 30316-30320.
Hoffmann N., Steinbüchel A. and Rehm B.H.A. 2000a. The Pseudomonas aeruginosa phaG gene product is involved in the synthesis of polyhydroxyalkanoic acid consisting of medium-chain-length constituents from non-related carbon sources. FEMS Microbiol. Lett. 184: 253-259.
Hoffmann N., Steinbüchel A. and Rehm B.H.A. 2000b. Homologous functional expression of cryptic phaG from Pseudomonas oleovorans establishes the transacylase-mediated polyhydroxy alkanoate biosynthetic pathway. Appl. Microbiol. Biotechnol. 54: 665-670.
Holms W.H. 1996. Flux analysis and control of the central metabolic pathways in Escherichia coli. FEMS Microbiol. Rev. 19: 85-116.
Hrabak O. 1992. Industrial production of ploy-ß-hydroxybutyrate. FEMS Microbiol. Rev. 103: 251-256.
Huijbert G.N.M., de Rijk T.C., deWaard P. and Eggink G. 1994. 13C Nuclear magnetic resonance studies of Pseudomonas putida fatty acid metabolic routes involved in poly(3-hydroxyalkanoate) synthesis. J. Bacteriol. 176: 1661-1666.
Kessler B. and Witholt B. 2001. Factors involved in the regulatory network of polyhydroxyalkanoate metabolism. J. Biotechnol. 86: 97-104.
Lee S.Y., Lee Y. and Wang F. 1999. Chiral compounds from bacterial polyesters: sugars to plastics to fine chemicals. Biotech. Bioeng. 65: 363-368.
Lee Y., Park S.H., Lim I.T., Han K. and Lee S.Y. 2000. Preparation of alkyl (R)-(—)3-hydroxybutyrate by acidic alcoholysis of poly-(R)-(—)-3-hydroxyl-butyrate. Enzyme Microb. Technol. 27: 33-36.
Madison L.L. and Huisman G.W. 1999. Metabolic engineering of poly(3-hydrocyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63: 21-53.
Magnuson K., Jackowshi S., Rock C.O. and Cronan J.E. Jr. 1993. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol. Rev. 57: 522-542.
Miller G.L. 1959. Use of dinitrosalicylic acid reagent for determi-nation of reducing sugar. Anal. Chem. 31: 426
Qi Q., Steinbüchel A. and Rehm B.H.A. 1998. Metabolic routing towards polyhydroxyalkanoic acid synthesis in recombinant Escherichia coli (fadR ): inhibition of fatty acid ß-oxidation by acrylic acid. FEMS Microbiol. Lett. 167: 89-94.
Ren Q., Sierro N., Witholt B. and Kessler B. 2000. FabG, an NADPH-dependent 3-ketoacyl reductase of Pseudomonas aeruginosa, provides precursors for medium-chain-length poly-3-hydroxyalkanoate biosynthesis in Escherichia coli. J. Bacteriol. 182: 2978-2981.
Rehm B.H.A., Krüger N. and Steinbüchel A. 1998. A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis. J. Biol. Chem. 273: 24044-24051.
Rehm B.H.A. and Steinbüchel A. 1999. Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int. J. Biol. Macromol. 25: 3-19.
Rehm B.H.A., Mitsky T.A. and Steinbüchel A. 2001. Role of fatty acid de novo biosynthesis in polyhydroxyalkanoic acid (PHA) and rhamnolipid synthesis by pseudomonads: establishment of the transacylase (PhaG)-mediated pathway for PHA biosynthesis in Escherichia coli. Appl. Environ. Microbiol. 67: 3102-3109.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zheng, Z., Zhang, MJ., Zhang, G. et al. Production of 3-hydroxydecanoic acid by recombinant Escherichia coli HB101 harboring phaG gene. Antonie Van Leeuwenhoek 85, 93–101 (2004). https://doi.org/10.1023/B:ANTO.0000020275.23140.ca
Issue Date:
DOI: https://doi.org/10.1023/B:ANTO.0000020275.23140.ca