Abstract
Purpose. The objective of this study was to develop a family of compartmental models to describe in a strictly quantitative manner the transdermal iontophoretic transport of drugs in vitro.
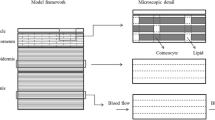
Methods. Two structurally different compartmental models describing the in vitro transport during iontophoresis and one compartmental model describing the in vitro transport in post-iontophoretic period are proposed. These models are based on the mass transfer from the donor compartment to the acceptor compartment via the skin as an intermediate compartment. In these models, transdermal iontophoretic transport is characterized by 5 parameters: 1) kinetic lag time (t L), 2) steady-state flux during iontophoresis (J ss), 3) skin release rate constant (K R), 4) the first-order rate constant of the iontophoretic driving force from the skin to the acceptor compartment (I 1), and 5) passive flux in the post-iontophoretic period (J pas). The developed models were applied to data on the iontophoretic transport in human stratum corneum in vitro of R-apomorphine after pretreatment with phosphate buffered saline pH 7.4 (PBS) and after pretreatment with surfactant (SFC), as well as the iontophoretic transport of 0.5 mg ml-1 rotigotine at pH 5 (RTG).
Results. All of the proposed models could be fitted to the transport data of PBS, SFC, and RTG groups both during the iontophoresis and in the post-iontophoretic period. The incorporation of parameter I 1 failed to improve the fitting performance of the model. This might indicate a negligible contribution of iontophoretic driving force to the mass transfer in the direction from the skin to the acceptor compartment, although it plays an important role in loading the skin with the drug. The estimated values of J ss of PBS, SFC, and RTG were identical (p > 0.05) to the values obtained with the diffusion lag time method. Moreover, time required to achieve steady-state flux can be estimated based on the parameter t L and the reciprocal value of parameter K R. In addition, accumulation of drug molecules in the skin is reflected in a reduction of the value of the K R parameter.
Conclusions. The developed in vitro models demonstrated their strength and consistency to describe the drug transport during and post-iontophoresis.
Similar content being viewed by others
REFERENCES
B. H. Sage. Iontophoresis. In E. W. Smith and H. I. Maibach (eds.), Percutaneous Penetration Enhancers, CRC Press, Boca Raton, 1995. pp. 351–368.
N. Kanikkannan, J. Singh, and P. Ramarao. In vitro transdermal iontophoretic transport of timolol maleate: effect of age and species. J. Control. Rel. 71:99–105 (2001).
S. Ganga and P. Ramarao. and J. Singh. Effect of Azone on the iontophoretic transdermal delivery of metoprolol tartrate through human epidermis in vitro. J. Control. Rel. 42:57–64 (1996).
S. Y. Oh, S. Y. Jeong, T. G. Park, and J. H. Lee. Enhanced transdermal delivery of AZT (Zidovudine) using iontophoresis and penetration enhancer. J. Control. Rel. 51:161–168 (1998).
K. C. Sung, J. Y. Fang, and H. Yoa-Pu. Delivery of nalbuphine and its prodrugs across skin by passive diffusion and iontophoresis. J. Control. Rel. 67:1–8 (2000).
J. Hirvonen and R. H. Guy. Iontophoretic delivery across the skin: electroosmosis and its modulation by drug substances. Pharm. Res. 14:1258–1263 (1997).
R. F. Lopez, M. V. Bentley, M. B. Delgado-Charro, and R. H. Guy. Iontophoretic delivery of 5-aminolevulinic acid (ALA): effect of pH. Pharm. Res. 18:311–315 (2001).
A. Luzardo-Alvarez, M. B. Delgado-Charro, and J. Blanco-Mendez. Iontophoretic delivery of ropinirole hydrochloride: ef-fect of current density and vehicle formulation. Pharm. Res. 18: 1714–1720 (2001).
M. B. Delgado-Charro, A. M. Rodriguez-Bayon, and R. H. Guy. Iontophoresis of nafarelin: effects of current density and concentration on electrotransport in vitro. J. Control. Rel. 35:35–40 (1995).
M. B. Delgado-Charro and R. H. Guy. Iontophoretic delivery of nafarelin across the skin. Int. J. Pharm. 117:165–172 (1995).
G. L. Li, M. Danhof, P. M. Frederik, and J. A. Bouwstra. Pre-treatment with a water-based surfactant formulation affects transdermal iontophoretic delivery of R-apomorphine in vitro. Pharm. Res. 20:653–659 (2003).
A. K. Nugroho, G. L. Li, M. Danhof, and J. A. Bouwstra. Transdermal iontophoresis of rotigotine across human stratum corneum in vitro: influence of pH and NaCl concentration. Pharm. Res. 21:844–850 (2004).
M. J. Pikal. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 46:281–305 (2001).
G. B. Kasting and J. C. Keister. Application of electrodiffusion theory for a homogenous membrane to iontophoretic transport through skin. J. Control. Rel. 8:195–210 (1989).
J. B. Phipps and J. R. Gyory. Transdermal ion migration. Adv. Drug Deliv. Rev. 9:137–176 (1992).
A. Jadoul, L. M. Dunbar, D. Ellis, and V. Preat. Modification induced on stratum corneum structure after in vitro iontophoresis: ATR-FTIR and X-ray scattering studies. J. Control. Rel. 42: 165–173 (1996).
A. Jadoul, J. A. Bouwstra, and V. Preat. Effects of iontophoresis and electroporation on the stratum corneum. Review of the biophysical studies. Adv. Drug Deliv. Rev. 35:89–105 (1999).
N. M. Volpato, P. Santi, and P. Colombo. Iontophoresis enhances the transport of acyclovir through nude mouse skin by electrorepulsion and electroosmosis. Pharm. Res. 12:1623–1627 (1995).
Pharsight Corporation. WinNonlin Professional v. 4.1 (2003).
J. Gabrielsson and D. Weiner. Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications. Swedish Pharmaceutical Press, Stockholm, Sweden, 2000.
M. Gibaldi and D. Perrier. Pharmacokinetics. Marcel Dekker, New York, 1982.
P. Singh, M. S. Roberts, and H. I. Maibach. Modeling of plasma levels of drugs following transdermal iontophoresis. J. Control. Rel. 33:293–298 (1995).
R. H. Guy, J. Hadgraft, and H. I. Maibach. A pharmacokinetic model for percutaneous absorbtion. Int. J. Pharm. 11:119–129 (1982).
R. H. Guy and J. Hadgraft. Transdermal drug delivery: a simplified pharmacokinetic approach. Int. J. Pharm. 24:267–274 (1985).
J. C. Shah. Application of kinetic model to in vitro percutaneous permeation of drugs. Int. J. Pharm. 133:179–189 (1996).
R. Van der Geest, M. Danhof, and H. E. Bodde. Iontophoretic delivery of apomorphine. I: In vitro optimization and validation. Pharm. Res. 14:1798–1803 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nugroho, A.K., Della Pasqua, O., Danhof, M. et al. Compartmental Modeling of Transdermal Iontophoretic Transport: I. In Vitro Model Derivation and Application. Pharm Res 21, 1974–1984 (2004). https://doi.org/10.1023/B:PHAM.0000048187.54125.ac
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000048187.54125.ac