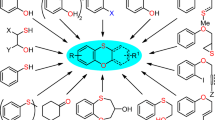
Abstract
1,5-Difluoro-2,4-dinitrobenzene starting material was treated via primary and/or secondary substitution with a variety of amino acids or amines and the aromatic m-dinitro groups were then reductively cyclized provide the 2-quinoxalinol analogs. The conditions for 1,5-dialkylamino-2,4-dinitrobenzene reduction have been systematically studied and optimized in solution. Three effective methods are described for the high-throughout generation of 2-quinoxalinol analogs.
Similar content being viewed by others
References
Liu, G., Zhang, L., Zhang, S. D., Yang, H. Z., Li, L., Wu, X. H., Sun G. C., Kou, B. B., Xu, S., Ji, Y. F. and Cheng, G. F., A novel and parallel approach for synthesis of 2-quinoxalinol analogs and their inhibition of TNF-α stimulation by LPS on macrophage in vitro, submitted.
Nicholas A. Meanwell, Herbert R. Roth, Edward C. R. Smith, Donald L. Wedding J. J. Kim Wright, J. Stuart Fleming and Elizabeth Gillespie, 1,3-Dihydro-2H-imidazo[4,5-b]quinolin-2-ones — inhibitors of blood platelet cAMP phosphodiesterase and induced aggregation, J. Med. Chem., 34 (1991) 2906–2916.
Kim, J. N., Lee, K. Y., Kim, H. S. and Kim, T. Y., Synthesis of 3-Ethoxycarbonyl-4-hydroxyquinoline N-Oxides from the Baylis-Hillman Adducts of o-Nitrobenzaldehydes, Org. Lett. (Communication), 2 (2000) 343–345.
Robert L. Wear and Cliff S. Hamilton, The synthesis of some quinoxaline derivatives, J. Am. Chem. Soc., 72(7) (1950) 2893–2894.
Norman H. Cromwell and Gerald D. Mercer, Amino derivatives of nitrochalcones. II.1 A new synthetic method for 3-aminoquinolines, J. Am. Chem. Soc., 79(23) (1957) 6201–6203.
Fox, B. A. and Threfall, T. L., 2,3-diaminopyridine, Org Synth., Coll., 5 (1973) 346–349.
Ono, A., Terasaki S. and Tsuruoka Y., Selective reduction of dinitrobenzenes, Chem. Ind. (Lond.), (1983) 477–478.
For reviews, see Rylander, P. N., Hydrogenation Methods, Ref. 497, p. 104, Catalytic Hydrogenation over Platinuim Metals, Academic Press, NY, 1967, p. 168.
Moody, C. J. and Pitts, M. R., Indium as a reducing agent: reduction of aromatic nitro groups, Synlett., 9 (1998) 1028.
Banik, B. K., Mukhopadhyay, C., Venkatraman, M. S. and Becker, F. F., A facile reduction of aromatic nitro compounds to aromatic amines by samarium and iodine, Tetrahedron Lett., 39 (1998) 7243–7246.
Fitch, R. W. and Luzzio, F. A., The aluminum amalgam reduction of 2-nitroalkanols promoted by ultrasound, Tetrahedron Lett., 35 (1994) 6013–6016.
Brinkman, H. R., The reduction of nitrobenzenes by triethylsilane using Wilkinson's catalysts, Synth. Commun., 26 (1996) 973–980.
Terpko, M. O. and Heck, R. F., Palladium-catalyzed triethylammonium formate reduction. 3. Selective reduction of dinitroaromatic compounds, J. Org. Chem., 45 (1980) 4992–4993.
Ram, S. and Ehrenkaufer, R. E., A general procedure for mild and rapid reduction of aliphatic and aromatic nitro compounds using ammonium formate as a catalytic hydrogen transfer agent, Tetrahedron Lett., 25 (1984) 3415–3418.
Barrett, A. G. M. and Spilling, C. D., Transfer hydrogenation: a stereospecific method for the conversion of nitro alkanes into amines, Tetrahedron Lett., 29 (1988) 5733–5734.
Entwistle, I. D., Jackson, A. E., Johnstone R. A. W. and Telford, R. P., Reduction of nitro-compound, J. Chem. Soc. Perkin Trans., 1 (1977) 443–444.
Petrini, M., Ballini, R. and Rosini, G., Reduction of aliphatic and aromatic nitro compounds with sodium borohydride in tetrahydrofuran using 10% palladium-on-carbon as catalyst, Synthesis, (1987) 713–714.
Ayyangar, N. R., Lugade, A. G., Nikrad, P. V. and Sharma, V. K., Catalytic reduction of nitroarenes with hydrazine hydrate in suitable solvents, Synthesis, (1981) 640–643.
Ayyangar, N. R., Kalkote, U. R. Lugad, A. G., NiKrad, P. V. and Sharma, V. K., Partial reduction of dinitroarenes to nitroanilines with hydrazine hydrate, Bull. Chem. Soc. Jpn., 56 (1983) 3159–3164.
Romanelli, M. G. and Beckers, E. I., Ethyl p-Dimethylaminophenylacetate, Org Synth, coll., 5 (1973) 552–554.
He, Y., Zhao, H., Pan, X. F. and Wang, S. F., Reduction with metal borohydride-transition metal salt system. I. Redction of aromatic nitro compounds with potassium borohydride-copper (I) chloride, Synth. Commun., 19 (1989) 3047–3050.
Hanaya, K., Muramatus, T., Kudo, H. and Chow, Y. L., Reduction of aromatic nitro-compounds to amines with sodium borohydridecopper(II) acetylacetonate, J. Chem. Soc. Perkin Trans., 1 (1979) 2409–2410.
Gillespie, H. B., Spano, F. and Graff, S., Synthesis of some substituted benzimidazoles, benzotriazoles, and quinoxalines, 25 (1960) 942–944.
For a review of the Zinin reduction, see Porter, H.K., Org. React., 20 (1973) 455.
Hughes, I., Application of polymer-bound phosphonium salts as traceless supports for solid phase synthesis, Tetrahedron Lett., 37 (1996) 7595–7598.
Babler, J. H. and Sarussi, S. J., Non-catalyzed reductions with formate salts: conversion of nitroaromatic compounds to the corresponding primary amines, Synth. Commun., 11 (1981) 925–930.
Baik, W., Han, J. l., Lee, K. C., Lee, N. H., Kim, B. H. and Hahn, J. T., Selective reduction of aromatic nitro compounds to aromatic amines by baker's yeast in basic solution, Tetrahedron Lett., 35 (1994) 3965–3966.
Wei, G. P. and Phillips, G. B., Solid phase synthesis of benzimidazolones, Tetrahedron Lett., 39 (1998) 179–182.
Mayer, J. P., Zhang, J. W., Bjergarde, K., Lenz, D. M. and Gaudino, J. J., Solid phase synthesis of 1,4-benzodiazepine-2,5-diones, Tetrahedron Lett., 37 (1996) 8081–8084.
Meyer, H. V., Dilley, G. J., Durgin, T. L., Powers, T. S., Winssinger, N. A., Zhu, W. and Pavia, M. R., Multiple simultaneous synthesis of phenolic libraries, Molecular Diversity, 1 (1995) 13–20.
Xing, W. K. and Ogata, Y., Steric acceleration by ortho substituent of the stannous chloride reduction of nitrobenzenes in aqueous ethanol, J. Org. Chem., 47 (1982) 3577–3581.
Inoue, H., Konda, M., Hashiyama, T., Otsuka, H., Watanabe, A., Gaino, M., Takahashi, H., Date, T., Okamura, K., Takeda, M., Narita, H., Murata, S., Odawara. A., Sasaki, H. and Nagao, T., Synthesis and biological evaluation of alkyl, alkoxy, alkylthio, or Amino-substituted 2,3-dihydro-1,5-benzothiazepin-4(5H)-ones, Chem. Pharm. Bull., 45 (1997) 1008–1026.
Bellamy, F. D. and Ou, K. Selective reduction of nitro compounds with stannous chloride in non acidic and non aqueous medium, Tetrahedron Lett., 25 (1984) 839–842.
Liu, G., Fan, Y. M., Calson, J. R. and Lam, K. S., Solution-phase synthesis of 1,5-dialkylamino-2,4-dinitrobenzene library and the identification of novel antibacterial compounds from this library, J. Combi Chem., 2(5) (2000) 467–474.
R. J. Booth and J. C. Hodges, Polymer-support quenching reagents for parallel purification, J. Am. Chem. Soc., 119 (1997) 4882–4886.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, XH., Liu, G., Zhang, J. et al. Solution-phase reductive cyclization of 2-quinoxalinol analogs: Systematic study of parallel synthesis. Mol Divers 8, 165–174 (2004). https://doi.org/10.1023/B:MODI.0000025639.89179.60
Issue Date:
DOI: https://doi.org/10.1023/B:MODI.0000025639.89179.60