Abstract
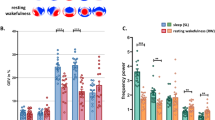
The present study explored EEG correlates of dream recall in 17 symptomatic, unmedicated depressed patients and in 19 healthy adults. EEG segments from the last 30 minutes of sleep, from the five minutes following morning awakening, and the absolute difference between sleep and waking EEG were contrasted between the two groups of participants during successful dream recall and during no recall. Period amplitude analysis was used to quantify EEG frequencies. Increased high-frequency beta incidence in the right hemisphere and amplitude in both hemispheres during sleep were associated with dream recall in both patient and control groups. Depressed patients also showed higher delta amplitude in both hemispheres during sleep associated with recall, but this effect did not reach significance. With regard to the changes between sleep and wakefiilness, a smaller change in right hemisphere beta and delta incidence characterized successful recall in healthy controls. By contrast, those with depression showed recall success when the sleep/wake shifts in right hemisphere beta and delta incidence were larger. Recall failure was characterized by small EEG shifts from sleep to wakefulness in the depressed group. The same effects were observed for beta and delta amplitude measures, except that healthy controls showed a large shift in delta amplitude in the sleep-wake transition during successful recall but not during recall failure. Recall in those with depression was associated with a dramatic shift in left hemisphere delta amplitude. These findings provide support for Koukkou and Lehmann's (1983, 1993) state-shift hypothesis of dream recall in healthy controls (except for left hemisphere delta amplitude) but not in the depressed. It appears that in order to recall a dream, depressed patients must undergo larger shifts in brain activity and perhaps a different pattern of reorganization of EEG frequencies than controls. This finding may account for the low rates of recall reported previously in this clinical group.
Similar content being viewed by others
REFERENCES
Antrobus, J., Ehrlichman, H., & Weiner, M. (1978). EEG asymmetry during REM and NREM: failure to replicate. Sleep Research, 7, 24.
Armitage, R. (1980). Changes in dream content as a function of time of night, stage of awakening, and frequency of dream recall. Unpublished Masters thesis, Carleton University, Ottawa.
Armitage, R. (1995). Microarchitectural findings in sleep EEG in depression: Diagnostic implications. Biological Psychiatry, 37, 72–84.
Armitage, R., & Hoffmann, R. (1997). Sleep electrophysiology of major depressive disorders. Current Review of Mood and Anxiety Disorders, 1, 139–151.
Armitage, R., Hoffmann, R., Loewy, D., & Moffitt, A. (1989). Variations in period-analysed EEG asymmetry in REM and NREM sleep. Psychophysiology, 26(3), 329–336.
Armitage, R., Hoffmann, R., & Moffitt, A. (1992a). Interhemispheric EEG activity in sleep and wakefulness: Individual differences in the basic rest-activity cycle (BRAC). In J. S. Antrobus & M. Bertini (Eds.), The Neuropsychology of Sleep and Dreaming (pp. 17–45). Hillsdale: Lawrence Erlbaum & Associates.
Armitage, R., Rochlen, A., Fitch, T., Trivedi, M., & Rush, A. J. (1995). Dream recall and major depression: A preliminary report. Dreaming, 5(3), 189–198.
Armitage, R., Roffwarg, H. P., Rush, A. J., Calhoun, J. S., Purdy, D. G., & Giles, D. E. (1992b). Digital period analysis of sleep EEG in depression. Biological Psychiatry, 31, 52–68.
Armitage, R., Rush, A. J., Trivedi, M., Cain, J., & Roffwarg, H. P. (1994). The effects of nefazodone on sleep architecture in depression. Neuropsychopharmacology, 10, 123–127.
Aserinsky, E., & Kleitman, N. (1953). Regular occurring periods of eye motility and concomitant phenomena during sleep. Science, 118, 273–274.
Dement, W. C., & Kleitman, N. (1957a). Cyclic variations in EEG during sleep and their reaction to eye movements, body motility, and dreaming. Electroencephalography and Clinical Neurophysiology, 9, 673–690.
Dement, W. C., & Kleitman, N. (1957b). Relation of eye movements during sleep to dream activity: an objective method for study of dreaming. Journal of Experimental Psychology, 53, 339–346.
Fiss, H. (1979). Current dream research: A psychobiological perspective. In B. Wolman (Ed.), Handbook of Dreams: Research, Theories And Applications (pp. 20–74). New York: Van Nostrand Rheinhold.
Foulkes, D. (1962). Dream reports from different stages of sleep. Journal of Abnormal Psychology, 65, 14–25.
Foulkes, D. (1996). Dream Research: 1953–1993. Sleep, 19(8), 609–624.
Goldstein, L., Stolzfus, N., & Gardocki, J. (1972). Changes in interhemispheric amplitude relations in the EEG during sleep. Physiology and Behavior, 8, 811–815.
Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry, 23, 56–62.
Hobson, J. A. (1988). The Dreaming Brain. New York: Basic Books.
Hobson, J. A., & McCarley, R. W. (1977). The brain as a dream state generator: An activation-synthesis hypothesis of the dream process. American Journal of Psychiatry, 134, 1335–1348.
Hoffmann, R., Moffitt, A., Wells, R., Sussman, P., Pigeau, R., & Shearer, J. (1984). Quantitative description of sleep stage electrophysiology using digital period analytic techniques. Sleep, 7(4), 356–364.
Hoffmann, R. F., Moffitt, A. R., Shearer, J. C., Sussman, P. S., & Wells, R. B. (1979). Conceptual and methodological considerations towards the development of computer-controlled research on the electro-physiology of sleep. Waking and Sleeping, 3, 1–16.
Koukkou, M., & Koukkou, D. (1993). A model of dreaming and its functional significance: The state-shift hypothesis. In A. Moffitt, M. Kramer, & R. Hoffmann (Eds.), The Functions of Dreaming (pp. 51–118). New York: State University Press.
Koukkou, M., & Lehmann, D. (1983). Dreaming: The functional state-shift hypothesis. A neuropsychophysiological model. British Journal of Psychiatry, 142, 221–231.
Moffitt, A., Hoffmann, R., Wells, R., Armitage, R., Pigeau, R., & Shearer, J. (1982). Individual differences among pre-and post-awakening EEG correlates of dream reports following arousals from different stages of sleep. Psychiatric Journal of the University of Ottawa, 7(2), 111–125.
Morel, C. R., Hoffmann, R. F., & Moffitt, A. R. (1991). The electrophysiological correlates of dream recall and nonrecall from stage 2 sleep. Canadian Journal of Psychology, 45(2), 140–147.
Pigeau, R. A., Hoffmann, R. F., & Moffitt, A. R. (1981). A multivariate comparison between two EEG analysis techniques: Period analysis and fast Fourier transform. Electroencephalography and Clinical Neurophysiology, 52, 656–658.
Pivik, T., & Foulkes, D. (1968). NREM mentation: Relation to personality, orientation time, and time of night. Journal of Consulting and Clinical Psychology, 32, 144–151.
Riemann, D., Low, H., Schredl, M., Wiegand, M., Dippel, B., & Berger, M. (1990). Investigations of morning and laboratory dream recall and content in depressive patients during baseline conditions and under antidepressive treatment with trimipramine. Psychiatric Journal of the University of Ottawa, 15(2), 93–99.
Riemann, D., Wiegand, M., Majer Trender, K., Dippel, B., & Berger, M. (1988). Dream recall and dream content in depressive patients, patients with anorexia nervosa and normal controls. In W. P. Koella, F. Obal, H. Schulz, & P. Visser (Eds.), Sleep '86 (pp. 373–375). Stuttgart: Gustav Fischer Verlag.
Spitzer, R. L., Williams, J. B. W., & Gibbons, M. (1986). The Structured Clinical Interview for DMS-III-R (SCID). New York: New York State Psychiatric Research Institute.
Tracy, R. I., & Tracy, L. N. (1974). Reports of mental activity from stages 2 and 4. Perceptual and Motor Skills, 38, 647–648.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rochlen, A., Hoffmann, R. & Armitage, R. EEG Correlates of Dream Recall in Depressed Outpatients and Healthy Controls. Dreaming 8, 109–123 (1998). https://doi.org/10.1023/B:DREM.0000005901.68193.1f
Issue Date:
DOI: https://doi.org/10.1023/B:DREM.0000005901.68193.1f