Abstract
1. The objective of the present study was to distinguish if inhibition of neuronal activity by hypoxia is related to a block of voltage-gated Na+ channels.
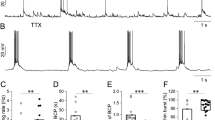
2. The effect of chemical hypoxia induced by cyanide (0.5 mM, 10 min perfusion) was studied with patch-clamp technique in visualized intact CA1 pyramidal neurons in rat brain slices. Action potentials were elicited in whole cell current-clamp recordings and the threshold was estimated by current pulses of 50-ms duration and incremental amplitudes (n = 31). The effect of cyanide on the Na+ current and conductance was studied in voltage clamp recordings from cell-attached patches (n = 13).
3. Cyanide perfusion during 10 min increased the threshold for excitation by 73 ± 79 pA (p = 0.001), which differed from the effect in control cells (11 ± 41 pA, ns). The change in current threshold was correlated to a change in membrane potential (r = −0.88, p < 0.0001). Cyanide had no significant effect on the peak amplitude, duration, or rate of rise of the action potential.
4. Cyanide perfusion did not change the Na+ current size, but caused a small decrease in E Na (−17 ± 22 mV, ns) and a slight increase in Na+ conductance (+14 ± 26%, ns), which differed (p = 0.045) from controls (−19 ± 23 %, ns).
5. In conclusion, chemical hypoxia does not cause a decrease in Na+ conductance. The decreased excitability during hypoxia can be explained by an increase in the current threshold, which is correlated with the effect on the membrane potential.
Similar content being viewed by others
REFERENCES
Arabadzisz, D., Ylinen, A., and Emri, Z. (2002). Increased inter-spike intervals and fast afterhyperpolarization of action potentials in rat hippocampal pyramidal cells accompanied with altered calbindin immunoreactivity 10-12 months after global forebrain ischemia. Neurosci. Lett. 331:103–106.
Baker, P. F., Hodgkin, A. L., and Shaw, T. I. (1962). The effects of changes in internal ionic concentrations on the electrical properties of perfused giant axons. J. Physiol. (Lond.) 164:355-374.
Belousov, A. B., Godfraind, J.-M., and Krnjevic, K. (1995). Internal Ca2+ stores involved in anoxic responses of rat hippocampal neurons. J. Physiol. 486:547–556.
Cummins, T. R., Donnelly,D.F., and 66:1471–1482.
Cummins, T. R., Jiang, C., and Haddad, G. G. (1993). Human neocortical excitability is decreased during anoxia via sodium channel modulation. J. Clin. Invest. 91:608–615.
Doolette, D. J., and Kerr, D. I. (1995). Hyperexcitability in CA1 of the rat hippocampal slice following hypoxia or adenosine. Brain Res. 677:127–137.
Englund, M., Hyllienmark, L., and Brismar, T. (2001). Chemical hypoxia in hippocampal pyramidal cells affects membrane potential differentially depending on resting potential. Neuroscience 106:89–94.
Erdemli,G., Xu, Y. Z., and Krnjevic, K. (1998). Potassium conductance causing hyperpolarization of CA1 hippocampal neurons during hypoxia. J. Neurophysiol. 80:2378–2390.
Fleidervish, I. A., Gebhardt, C., Astman, N., Gutnick, M. J., and Heinemann, U. (2001). Enhanced spontaneous transmitter release is the earliest consequence of neocortical hypoxia that can explain the disruption of normal circuit function. J. Neurosci. 21:4600–4608.
Fujimura, N., Tanaka, E., Yamamoto, S., Shigemori, M., and Higashi, H. (1997). Contribution of ATPsensitive potassium channels to hypoxic hyperpolarization in rat hippocampal CA1 neurons in vitro. J. Neurophysiol. 77:378–385.
Fujiwara, N., Higashi, H., Shimoji, K., and Yoshimura, M. (1987). Effects of hypoxia on rat hippocampal neurones in vitro. J. Physiol. 384:131–151.
Grondahl, T. O., and Langmoen, I. A. (1996). Cytotoxic effects of Ca2+ released from intracellular stores during cerebral energy deprivation. Neurol. Res. 18:499–504.
Gu, X. Q., and Haddad, G. G. (2001). Decreased neuronal excitability in hippocampal neurons of mice exposed to cyclic hypoxia. J. Appl. Physiol. 91:1245-1250.
Hammarstr öm, A. K. M., and Gage, P.W. (1998). Inhibition of oxidative metabolism increases persistent sodium current in rat CA1 hippocampal neurons. J. Physiol. 510(3):735–741.
Hammarstr öm, A. K. M., and Gage, P. W. (2000). Oxygen-sensing persistent sodium channels in rat hippocampus. J. Physiol. 529(1):107–118.
Hansen, A. J., Hounsgaard, J., and Jahnsen, H. (1982). Anoxia increases potassium conductance in hippocampal nerve cells. Acta physiol. Scand. 115:301–310.
Hille, B. (1992). Ionic Channels of Excitable Membranes, 2nd edn., Sinauer Associates Inc., Sunderland, MA, pp. 462–468.
Hodgkin, A. L., and Huxley, A. F. (1952a). Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. (Lond.) 116:449–472.
Hodgkin, A. L., and Huxley, A. F. (1952b). A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. (Lond.) 117:500–544.
Horn, E. M., and Waldrop, T. G. (2000). Hypoxic augmentation of fast-inactivating and persistent sodium currents in rat caudal hypothalamic neurons.J. Neurophysiol. 84:2572–2581.
Hyllienmark, L., and Brismar, T. (1996). Effect of metabolic inhibition on K+ channels in pyramidal cells of the hippocampal CA1 region in rat brain slices. J. Physiol. 496:155–164.
Hyllienmark, L., and Brismar,T. (1999). Effect of hypoxia on membrane potential and resting conductance in rat hippocampal neurons. Neuroscience 91:511–517.
Isom, G. E., and Way, J. L. (1984). Effects of oxygen on the antagonism of cyanide intoxication: Cytochrome oxidase, in vitro. Toxicol. Appl. Pharmacol. 74:57–62.
Jensen, F. E., Wang, C., Stafstrom, C. E., Liu, Z., Geary, C., and Stevens, M. C. (1998). Acute and chronic increase in excitability in rat hippocampal slices after perinatal hypoxia in vivo. J. Neurophysiol. 79:73–81.
Khodorov, B. I., and Timin, E. N. (1975). Nerve impulse propagation along uniform fibers. Progr. Biophys. Mol. Biol. 30:145–84.
Kulik, A., Brockhaus, J., Pedarzani, P., and Ballanyi, K. (2002). Chemical anoxia activates ATP-sensitive and blocks Ca2+-dependent K+ channels in rat dorsal vagal neurons in situ. Neuroscience 110:541–554.
Leblond, J., and Krjnevic, K. (1989). Hypoxic changes in hippocampal neurons. J. Neurophysiol. 62:1–14.
Mazza, E., Jr., Edelman, N. H., and Neubauer, J. A. (2000). Hypoxic excitation in neurons cultured from the rostral ventrolateral medulla of the neonatal rat. J. Appl. Physiol. 88:2319–2329.
O'Reilly, J. P., Cummins, T. R., and Haddad, G. G. (1997). Oxygen deprivation inhibits Na+ current in rat hippocampal neurones via protein kinase C. J. Physiol. 503:479–488.
Reiner, P. B., Laycock, A. G., and Doll, C. J. (1990). A pharmacological model of ischemia in the hippocampal slice. Neurosci. Lett. 119:175–178.
Schiff, S. J., and Somjen, G. G. (1987). The effect of graded hypoxia on the hippocampal slice: An in vitro model of the ischemic penumbra. Stroke 18:30–37.
Semenov, D. G., Samoilov, M. O., Zielonka, P., and Lasarewicz, J. W. (2000). Responses to reversible anoxia of intracellular free and bound Ca2+ in rat cortical slices. Resuscitation 44:207–214.
Stuart, G. J., Dodt, H.-U., and Sakmann, B. (1993). Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Arch. 423:511–518.
Xia, Y., Fung, M. L., O'Reilly, J. P., and Haddad, G. G. (2000). Increased neuronal excitability after long-term O(2) deprivation is mediated mainly by sodium channels. Mol. Brain Res. 76:211–219.
Yamamoto, S., Tanaka, E., and Higashi, H. (1997).Mediation by intracellular calcium-dependent signals of hypoxic hyperpolarization in rat hippocampal CA1 neurons in vitro. J. Neurophysiol. 77:386–392.
Zhang, L., and Krnjevic, K. (1993). Whole-cell recording of anoxic effects on hippocampal neurons in slices. J. Neurophysiol. 69:118–127.
Zhu, P. J., and Krnjevic, K. (1999). Persistent block of CA1 synaptic function by prolonged hypoxia. Neuroscience 90:759–770.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Englund, M., Bjurling, M., Edin, F. et al. Hypoxic Excitability Changes and Sodium Currents in Hippocampus CA1 Neurons. Cell Mol Neurobiol 24, 685–694 (2004). https://doi.org/10.1023/B:CEMN.0000036405.53992.78
Issue Date:
DOI: https://doi.org/10.1023/B:CEMN.0000036405.53992.78