Abstract
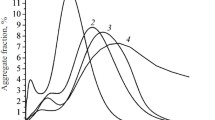
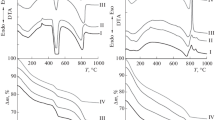
Thermal studies, sometimes together with X-ray analysis, were applied to investigate the process of hydration in the systems calcium silicates (tricalcium silicate or dicalcium silicate) - water - electrolyte. Alkali metal or alkaline-earth metal salts were used as electrolytes. Results and conclusions are presented concerning the action of electrolytes upon the kinetics of hardening of the calcium silicates and the composition and phase transformation of calcium hydrosilicate in the presence of low proportions of electrolytes (0.5, 2 and 5 mass%), these effects being due to ionic substitution. A higher proportion of electrolyte (above 2%) in the systems calcium silicate-water can determine the formation of a complex salt, e.g. calcium hydroxysalts or double hydrosilicates.
Similar content being viewed by others
REFERENCES
W. Richartz, Zement Kalk Gips, 22 (1969) 447.
V. S. Ramachandran, RILEM. Mat. Constr. Materials Struct., 4 (1971) 3.
M. Muntean, ‘The hardening process in the binder—electrolyte—water systems’, Thesis, Polytechnic Institute Bucharest 1972, p. 27.
I. Teoreanu and M. Muntean, Silicat Ind., 39 (1974) 49.
I. Teoreanu and M. Muntean, Proced. VIth Intern. Congr. Chem. Cem., Moscow, 1974, p. 51.
V. S. Ramachandran and Z. Chung-Mei, Il Cemento, 83 (1986) 129.
L. Montanaro, A. Negro and M. Regourd, Cem. Concr. Res., 18 (1988) 431.
I. Teoreanu and M. Muntean, Mat. Const. (Romania), 20 (1990) 101.
H. F. W. Taylor, ‘Chemistry of Cements’, Academic Press, London 1990.
V. S. Ramachandran, J. J. Beaudoin, S. L. Sarkar and Xu Almin, Il Cemento, 90 (1993) 73.
A. Tagnit and S. Bouraaoui, Proceed. Xth Intern. Congr. Chem. Cem., Goyheburg-Sweden, Vol. 2 (1997) p. 8.
L. Chen, L. Schzong and W. Yanrong, Proceed. IVth Beijing Intern. Symp. Cem. and Concr., Intern. Acad. Publishers Vol. 1, (1998) p. 307.
S. Tsivilis, G. Kakali, E. Chaniotakis and A. Souvaridou, J. Therm. Anal. Cal., 52 (1998) 863.
S. Monosi, I. Alvera and M. Collepardi, Il Cemento, 86 (1989) 97.
I. Teoreanu, M. Georgescu and G. Scaunasu, Rev. Roum. Chim., 16 (1971) 861.
Joint Comm. Diffr. Stand., Powder Diffraction., File Inorganic Phases Sets 1–5 Swarthmore, PA, USA 1974, p. 207.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Teoreanu, I., Muntean, M. Thermal Studies of the Hardening of the Systems Calcium Silicates–Electrolyte–Water. Journal of Thermal Analysis and Calorimetry 62, 809–819 (2000). https://doi.org/10.1023/A:1026741913366
Issue Date:
DOI: https://doi.org/10.1023/A:1026741913366