Abstract
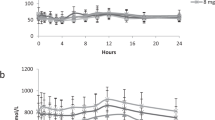
We characterized the effect of orthotopic liver transplantation on the catabolism of branched-chain L-amino acids in a female patient with classical form of maple syrup urine disease. Transplantation was performed at the age of 7.4 years due to a terminal liver failure triggered by a hepatitis A infection. Since then, the patient is on an unrestricted diet and plasma concentrations of branched-chain L-amino and 2-oxo acids are stable, yet at moderately increased levels (2- to 3-fold of control). L-Alloisoleucine concentrations, however, remained remarkably elevated (>5-fold of control). In vivo catabolism was investigated by measuring the metabolic L-alloisoleucine clearance and whole-body leucine oxidation in the postabsorptive state. In an oral loading test with 580 μmol alloisoleucine per kg body wt, the L-alloisoleucine elimination rate constant (0.067 h−1) was in the normal range (0.069±0.012 h−1, n=4). In an oral L-[1-13C]leucine load (38 μmol/kg body wt), 19.5% of the tracer dose applied was recovered in exhaled 13CO2 versus 18.9±3.6% in healthy subjects (n=10). Thus, the patient exhibited obviously normal whole-body catabolic rates although branched-chain L-amino acid oxidation was confined to the liver transplant. Most likely, the enhanced substrate supply from extrahepatic sources led to an elevation of the plasma concentrations and thus induced a compensatory enhancement of the metabolic flux through the branched-chain 2-oxo acid dehydrogenase complex in the intact liver tissue.
Similar content being viewed by others
REFERENCES
Bodner A, Hammen HW, Renn W, Wendel U, Schadewaldt P (1997) Whole body branched-chain L-amino acid oxidation in overnight fasted human subjects. Isotopes Environ Health Stud 33: 189–196.
Bodner A, Wendel U, Saudubray J-M, Schadewaldt P (1998) Liver transplantation in classical maple syrup urine disease (MSUD): near normalization of branched-chain L-amino acid metabolism. J Inherit Metab Dis 21(supplement 2): 19.
Bowtell JL, Leese GP, Smith K, et al (1998) Modulation of whole body protein metabolism, during and after exercise, by variation of dietary protein. J Appl Physiol 85: 1744–1752.
Chuang DT, Shih VE (1995) Disorders of branched chain amino acid and keto acid metabolism. In Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Mol-ecular Bases of Inherited Disease, 7th edn. New York: McGraw-Hill, 1239–1277.
Danner DJ, Davidson ED, Elsas LJ (1975) Thiamine increases the specific activity of human liver branched-chain a-ketoacid dehydrogenases. Nature 254: 529–530.
Felig P (1975) Amino acid metabolism in man. Annu Rev Biochem 44: 933–955.
Goto M, Shinno H, Ichihara A (1977) Isoenzyme patterns of branched-chain amino acid transaminase in human tissues and tumors. Gann 68: 663–667.
Haycock G, Schwartz G, Wisotsky D (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children and adults. J Pediatr 93: 62–66.
Huang YS, Chuang DT (1999) Down-regulation of rat mitochondrial branched-chain 2-oxoacid dehydrogenase kinase gene expression by glucocorticoids. Biochem J 339: 503–510.
Jackman ML, Gibala MJ, Hultman E, Graham TE (1997) Nutritional status affects branched-chain oxoacid dehydrogenase activity during exercise in humans. Am J Physiol 272: E233-E238.
Kaplan P, Mazur AM, Smith R, et al (1997) Transplantation for maple syrup urine disease (MSUD) and methylmalonic acidopathy (MMA). J Inherit Metab Dis 20(supplement 1): 37.
Khatra BS, Chawla RK, Sewell CW, Rudman D (1977) Distribution of branched-chain a-keto acid dehydrogenases in primate tissues. J Clin Invest 59: 558–564.
Magnus-Levy A (1910) Über den Gehalt normaler menschlicher Organe an Chlor, Calcium, Magnesium und Eisen sowie an Wasser, Eiweiß und Fett. Biochem Z 24: 363–380.
Merinero B, Rerez-Cerda C, Sanz P, et al (1994) Liver transplantation (LT) in a spanish MSUD patient. Abstracts 32nd SSIEM Annual Symposium, P64.
Netter JC, Cossarizza G, Narcy C, et al (1994) Devenir á moyen terme de deux cas de leucinose: place de la transplantation hépatique dans le traitement. Arch Pédiatr 1: 730–734.
Paul HS, Liu WQ, Adibi SA (1996) Alteration in gene expression of branched-chain keto acid dehydrogenase kinase but not in gene expression of its substrate in the liver of clofibrate-treated rats. Biochem J 317: 411–417.
Paxton R, Harris RA (1984) Regulation of branched-chain a-keto acid dehydrogenase kinase. Arch Biochem Biophys 231: 48–57.
Rush JWE, MacLean DA, Hultman E, Graham TE (1995) Exercise causes branched-chain oxoacid dehydrogenase dephosphorylation but not AMP deaminase binding. J Appl Physiol 78: 2193–2200.
Schadewaldt P, Wendel U (1997) Metabolism of branched-chain amino acids in maple syrup urine disease. Eur J Pediatr 156(supplement 1): S62-S66.
Schadewaldt P, Hummel W, Trautvetter U, Wendel U (1989) A convenient enzymatic method for the determination of 4-methyl-2-oxopentanoate: comparison with high performance liquid chromatographic analysis. Clin Chim Acta 183: 171–182.
Schadewaldt P, Hammen HW, Dalle-Feste C, Wendel U (1990) On the mechanism of L-alloisoleucine formation: studies on a healthy subject and in fibroblasts from normals and patients with maple syrup urine disease. J Inherit Metab Dis 13: 137–150.
Schadewaldt P, Dalle-Feste C, Langenbeck U, Wendel U (1991) Oral L-alloisoleucine loading studies in healthy subjects and in patients with maple syrup urine disease. Pediatr Res 30: 430–434.
Schadewaldt P, Bodner A, Brösicke H, Hammen HW,Wendel U (1998a) obligate heterozygotes, and healthy subjects. Pediatr Res 43: 592–600.
Schadewaldt P, Wendel U, Bodner A (1998b) On the relation of leucine oxidation to total metabolic flux through the branched-chain 2-oxo acid dehydrogenase complex (BCOA-DH) in situ. J Inherit Metab Dis 21(supplement 2): 18.
Schadewaldt P, Bodner-Leidecker A, Hammen HW, Wendel U (1999) Significance of L-alloisoleucine in plasma for diagnosis of maple syrup urine disease. Clin Chem 45: 1734–1740.
Schadewaldt P, Bodner-Leidecker A, Hammen HW, Wendel U (2000) Formation of L-alloisoleucine in vivo: a L-[13C]isoleucine study in man. Pediatr Res 45: 271–277.
Shreeve WW, Cerasi E, Luft R (1970)Metabolism of [2–14C]pyruvate in normal, acromegalic, and HGH treated human subjects. Acta Endocrinol 65: 155–169.
Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM (1998)A mol-ecular model of human branched-chain amino acid metabolism. Am J Clin Nutr 68: 72–81.
Tessari P, Garibotto G, Inchiostro S, et al (1996) Kidney, splanchnic, and leg protein turnover in humans. Insight from leucine and phenylalanine kinetics. J Clin Invest 98: 1481–1492.
Torres N, Lopez G, De Santiago S, Hutson SM, Tovar AR (1998) Dietary protein level regulates expression of the mitochondrial branched-chain aminotransferases in rats. J Nutr 128: 1368–1375.
van Hall G, Saltin B, van der Vusse GJ, Söderlund K, Wagenmakers AJM (1995) Deamination of amino acids as source for ammonia production in human skeletal muscle during prolonged exercise. J Physiol 489: 251–261.
Wagenmakers AJM, Brookes JH, Coakley JF, Reilly T, Edwards RHT (1989) Exercise-induced activation of the branched-chain 2-oxo acid dehydrogenase in human muscle. Eur J Appl Physiol 59: 159–167.
Wendel U, Saudubray JM, Bodner A, Schadewaldt P (1999) Liver transplantation in maple syrup urine disease. Eur J Pediatr 158(supplement 1): S60-S64.
Wolfe RR (1984) Tracers in Metabolic Research: Radioisotope and Stable Isotope Mass Spectrometry Methods. New York: Wiley-Liss, 230–232.
Yeaman SJ (1989) The 2-oxo acid dehydrogenase complexes: recent advances. Biochem J 257: 625–632.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bodner-Leidecker, A., Wendel, U., Saudubray, JM. et al. Branched-chain L-amino acid metabolism in classical maple syrup urine disease after orthotopic liver transplantation. J Inherit Metab Dis 23, 805–818 (2000). https://doi.org/10.1023/A:1026708618507
Issue Date:
DOI: https://doi.org/10.1023/A:1026708618507