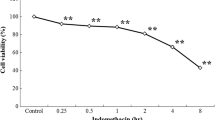
Abstract
Prostaglandins (PG) protect gastrointestinalcells against damage induced by ethanol (EtOH) and othernoxious agents, a process termed cytoprotection. Thepresent study investigated the relationships between microtubule (MT) stability, protein kinase C(PKC) activation, and calcium efflux as a possiblemechanism of PG's protective action using a humancolonic cell line (Caco-2) exposed to known damagingconcentrations of EtOH (7.5% and 10% ). Preincubation ofCaco-2 cells with 16,16-dimethyl-PGE2 (PG,2.6 μM) significantly increased PKC activity in thesecells. Pretreatment of Caco-2 cells with 50 μM OAG (asynthetic diacylglycerol and PKC activator) or 30 nM TPA(a direct PKC activator) prior to exposure to 7.5% or10% EtOH for 5 min significantly reduced cell injury, asdetermined by trypan blue exclusion, and increased MT stability, as confirmed by confocalmicroscopy. Pretreatment of Caco-2 cells with 4alpha-PDD (an inactive phorbol ester, 20 nM) failed toprevent cell injury and disruption of the MTcytoskeleton. Preincubation with staurosporine (a PKC inhibitor, 3 nM)abolished the protective effects of PG in cells exposedto 7.5% and 10% EtOH. Incubation of Caco-2 cells withA23187 (a Ca2+ ionophore), similar to 10%EtOH, caused a significant reduction in cell viability andMT stability. Preincubation with A23187 in combinationwith PG or OAG prior to subsequent exposure to EtOHsignificantly abolished the protective effects of PG or OAG pretreatment. Finally, pretreatmentwith OAG, TPA, or PG resulted in significant increasesin calcium-45 efflux, which correlated with increasedstability of the MT cytoskeleton. These data suggest that PG possesses direct protective effectsagainst EtOH injury in Caco-2 cells and may act bystabilizing MT through the PKC signal transductionpathway and/or stimulation of calcium efflux from thecells.
Similar content being viewed by others
REFERENCES
Arakawa T, Nakamdra A, Fukuda T, Satoh T, Kobayashi K: Proection of rat gastric cells by prostaglandins from damage caused by ethanol. Preliminary report. J Clin Gastroenterol 10( suppl):S28- S34, 1988
Barzilai A, Schiessel R, Kivilaakso E, Matthews JB, Fleischer LA, Bartzokis G, Silen W: Effect of 16, 16-dimethyl-prostaglandin E2 on ulceration of isolated amphibian gastric mucosa. Gastroenterology 78:1508–1512, 1980
Cherner JA, Naik L, Tarnawski A, Brzozowski T, Stachura J, Singh G: Ability of prostaglandins to reduceethanol injury to dispersed chief cells from guinea pig stomach. Am J Physiol 256:G704–G714, 1989
Konda Y, Nishisaki H, Nakano O, Matsuda K, Wads K, Nagao M, Matozaki T, Sakamoto C: Prostaglandin protects isolated guinea pig chief cells against ethanol injury via an increasein diacylglycerol. J Clin Invest 86:1897–1903, 1990
Tarnawski A, Brzozowski T, Sarfeh IJ, Krause WJ, Ulich TR, Gergely H, Hollander D: Prostaglandin protection of human isolated gastric glands against indomethacin and ethanol injury. J Clin Invest 81:1081–1089, 1988
Terano A, Mach T, Stachura J, Tarnawski A, Ivey KJ: Effect of 16,16-dimethyl-prostaglandin E2 on aspirin-induced damage to rat gastric epithelial cells in tissue culture. Gut 25:19–25, 1984
Terano A, Ota S, Mach T, Hiraishi H, Stachura J, Tarnawski A, Ivey KJ: Prostaglandin protects against taurocholate-induced damageto rat gastric mucosal cell culture. Gastroenterology 92:669–677, 1987
Robert A: Antisecretory, antiulcer, cytoprotective, and diarrheogenic properties of prostaglandins. In Advances in Prostaglandin and Thromboxane Research, Vol. II. B Samuelsson, R Paletti (eds). New York, Raven Press, 1976, pp 507–520
Robert A: Cytoprotection by prostaglandins. Gastroenterology 77:761–767, 1977
Whittle BJ, Vane JR: Prostanoids as regulators of gastrointestinal function. In Physiology of Gastrointestinal Tract, 3rd ed. LR Johnson (ed). New York, Raven Press, 1987, pp 143–180
Chaudhury TK, Jacobson ED: Prostaglandin cytoprotection of gastric mucosa. Gastroenterology 74:59–63, 1978
Miller TA: Protective effects of prostaglandins against gastric mucosal damage: Current knowledgeand proposed mechanisms. Am J Physiol 243:G601–G623, 1983
Robert A, Nezamis JE, Lancaster C, Hancher AJ: Cytoprotection by prostaglandins in rats: Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology 77:433–443, 1979
Banan A, Smith GS, Rickenberg CL, Kokoska ER, Miller TA: Protection against ethanol injury by prostaglandins in a human intestinal cell line: Roleof microtubules. Am J Physiol 274:G111–G121, 1998
Allen RD: The microtubuleas an intracellular engine. Sci Am 256( 2):42–49, 1987
Allen RD: Gliding movement of and bidirectional transport along singlenativemicrotubules from squid axoplasm: Evidence for an activeroleof microtubules in cytoplasmic transport. J Cell Biol 100:1736–1752, 1985
Alvia J: Microtubule Function. Life Sci 50:327–333, 1987
Bershadsky AD, Vasiliev JM: Pseudopodial activity at the active edge of migrating fibroblasts is decreased after drug induced microtubule depolymerization. Cell Motil Cytoskeleton 19:152–158, 1991
Bretscher MS: How animal cells move. Sci Am 257(6):72–90, 1987
Smith G, Hendrix MJC, Mercer DW, Miller TA: Prostaglandin E2 pre treatment attenuates 45calcium accumulation and microtubule disruption in cultured rat intestinal cells exposed to ethanol. Gastroenterology 110( 4) A194, 1996
Kishimoto A, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka DY: Activation of calcium and phospholipiddependent protein kinaseC, its possible relation to phosphatidylinositol turnover. J Biol Chem 255:2273–2276, 1980
Stenson WF, Easom RA, Riehl TE, Turk J: Regulation of paracellular permeability in Caco-2 cell monolayers by protein kinase C. Am J Physiol 265:G955–G962, 1993
Ota S, Hata Y, Terano A, Yoshiura K, Hiraishi H, Kawabe T, Mutoh H, Shina S, Sugimoto T: Roles of Ca21 and protein kinaseC in regulation of prostaglandin E2 releaseby cultured rabbit gastric epithelial cells. Dig Dis Sci 38:1426–1434, 1993
Tepperman BL, Tan SY, Whittle BJR: Effects of calciummodifying agents on integrity of rabbit isolated gastric mucosal cells. Am J Physiol 278 (Gastrointest Liver Physiol 24):G119–G127, 1991
Tepperman BL, Soper BD: Effect of extracellular Ca1 1 on indomethacin induced injury to rabbit dispersed gastric mucosal cells. Can J Pharmacol 72:63–69, 1994
Wong HM, Soper BD, Tepperman BL: Roleof calcium in thromboxaneB2 mediated injury to rabbit gastric mucosal cells. Dig Dis Sci 40(9):2022–2028, 1995
Phillips HJ: Dyeexclusion tests for cell viability. In Tissue CultureMethods and Applications. PF Krause, MK Patterson (eds). New York, Academic Press, 1973, pp 406–408
Katz A: Protein KinaseC activity measurements. Biochemistry 30:4334–4341, 1991
Bradford MA: A rapid and sensitivemethod for thequantitation of microgram quantities of protein utilizing theprinciple of dyebinding. Anal Biochem 72:224–254, 1976
Dormer RL, Pouslen JH, Licko V, Williams JA: Calcium fluxes in isolated pancreatic acini: Effects of secretagogues. Am J Physiol 240 (Gastrointest and Liver Physiol 3):G38–G49, 1981
Harter JL: Critical values for Dunnett's new multiplerange test. Biometrics 16:671–685, 1960
Arakawa T, Fukuda T, Kobyashi K, Tarnawski A: Prostaglandin-induced protection of cultured rat gastric cells against ethanol is inhibited by a microtubule inhibitor. Digestion 57:41–46, 1996
Luxford KA, Murph CR: Cytoskeletal control of theapical surface transformation of the rat uterine epithelium. Biol Cell 79:111–116, 1993
Parczyk K, Hasse W, Kondor-Koch C: Microtubules are involved in the secretion of proteins at the apical cell surface of polarized epithelial cell (MDCK). J Biol Chem 264:16837–16846, 1989
Blais A, Bissonette T, Berteloot A: Common characteristics for Na1-dependent sugar transport in Caco-2 and human fetal colon. J Membr Biol 99:113, 1988
Cohen MB, Jensen NJ, Hawkins JA: Receptors for Escherichia coli heat stable enterotoxin in human intestine and in a human intestinal cell line(Caco-2). J Cell Physiol 156:138–144, 1993
Dantzig AH, Tabas LB, Bergin L: Cephaclor uptake by the proton-dependent dipeptide transport carrier of human intestinal Caco-2 cells and comparison to cephalexin uptake. Biochim Biophys Acta 1112:167–173, 1992
Dix CJ, Hassan H, Obray HY, Stah R, Wilsomn G: The transport of vitamin B12 through polarized monolayers of Caco-2 cells. Gastroenterology 98:1272–1279, 1990
Gilbert T, Le Bivic A, Quaroni A, Rodreguez-Boulan E: Microtubule organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. J of Cell Biol 133( 2):275–288, 1991
Hauri HP, Sterchi EE, Bienz D, Fransen MA, Marxer A: Expression of intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol 101:838–851, 1985
Hoeflich A, Yang Y, Kessler U: Human colon carcinoma cells (Caco-2) synthesize IGF-II and express IGF-I receptors and IGF-II/M6P receptors. Mol Cell Endocrinol 101:141–150, 1994
Howell S, Kenny AJ, Turner AJ: A survey of membrane peptidases in two human colonic cell lines, Caco-2 and HT-29. Biochem J 284:595–601, 1992
Laburthe M, Rousset M, Rouyer-Fessard C: Development of vasoactive intestinal peptide-responsive adenylat ecyclase during enterocytic differentiation of Caco-2 cells in culture: Evidence for an increased receptor level. J Biol Chem 262:10180–10184, 1987
Mahraoui L, Rousset M, Ux E, Darmoul D, Zweibaum A, Brot-Laroche E: Expression and localization of GLUT-5 in Caco-2 cells, human small intestine, and colon. Am J Physiol 263 (Gastrointest Liver Physiol 26):G312–G318, 1992
Mahraoui L, Rodolosse A, Barbat A: Presenceand differential expression of SGLT1, GLUT1, GLUT2, GLUT3 and GLUT5 hexose-transporter mRNAs in Caco-2 cell clones in relation to cell growth and glucose consumption. Biochem J 298:629–633, 1994
Meunier VM, Bourrie Y, Berger Y, Fabre G: The human intestinal epithelial cell line Caco-2: Pharmacological and pharmecoke netics applications. Cell Biol Toxicol 11:187–194, 1995
Wallace JL, Whittle BJR: The use of in vivo and in vitro markers of cellular disruption to assess damage and protection in the gastrointestinal tract. In Ulcer Disease: New Aspects of Pathogenesis and Pharmacology. S Szabo, CJ Pfeiffer (eds). Boca Raton, Florida, CRC Press, 1989, pp 401–415
Nishizuka Y: The role of protein kinase C in cell surface signal transduction and tumor promotion. Nature(London) 308:693–695, 1984
Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka DY: Direct activation of calcium-activated phospholipiddependent protein kinase by tumor promoting phorbol ester. Biol Chem 257:7847–7851, 1982
Hori M, Kitakaze M, Iwai K, Takeda H, Inoue M, Kamada T: b-Adrenergic stimulation disassembles microtubules in neonatal rat cultured cardiomyocytes through intracellular Ca2+ overload. Circ Res 75:324–334, 1994
Kakiuchi S, Sobue K: Ca2+ and calmodulin-dependent flip-flop mechanism in microtubule assembly- disassembly. FEBS Lett 132:141–143, 1981
Schliwa M, Euteneuer U, Bulinski JC, Izant JG: Calcium lability of cytoplasmic microtubules and its modulation by microtubule-associated proteins. Proc Natl Acad Sci USA 78:1037–1041, 1981
Olge CW, Cho CH, Tong MC, Koo MWL: The influence of verapamil on the gastric effects of stress in rats. Eur J Pharmacol 112:399–404, 1985
Diziki AJ, Harmon JW, Batzri S, Blass BL, Ugrate RM: Role of 16,16-dmPGE 2 in gastric cell protection against bilesalts. In Mechanism of Injury, Protection, and Repair of theUpper Gastrointestinal Tract. A Garner, PO O'Brien (eds). John Wiley & Sons: Toronto, 1991, pp 61–70
Werth JL, Usachev YM, Thayer SA: Modulation of calcium efflux from rat dorsal root ganglion neurons. J Neurosci 16(3):1008–1015, 1996
Rights and permissions
About this article
Cite this article
Banan, A., Smith, G.S., Deshpande, Y. et al. Prostaglandins Protect Human Intestinal Cells Against Ethanol Injury by Stabilizing Microtubules (Role of Protein Kinase C and Enhanced Calcium Efflux). Dig Dis Sci 44, 697–707 (1999). https://doi.org/10.1023/A:1026649422607
Issue Date:
DOI: https://doi.org/10.1023/A:1026649422607