Abstract
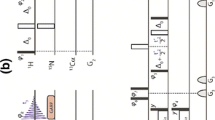
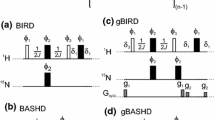
NMR studies of protein structures require knowledge of spectral assignments through correlation spectroscopy and the measurement of dipolar interactions by NOESY-type experiments. In order to obtain NOEs for protons with degenerate chemical shifts, which is particularly common for large proteins with significant helical content, 3D and 4D 15N/15N separated NOESY experiments (HSQC-NOESY-HSQC) are essential for NMR studies of these proteins. TROSY sections could replace the latter or both HSQC parts of the 3D and 4D 15N/15N separated HSQC-NOESY-HSQC pulse sequences to enhance signal sensitivity and improve resolution. For a 1.0 mM, 100% 15N and 70% 2H-labeled Trichosanthin sample (∼27 kDa) at 5 °C it is found that sensitivity enhancements could only be obtained when TROSY sections replace the latter HSQC parts of 3D and 4D 15N/15N separated HSQC-NOESY-HSQC pulse sequences. The sensitivities of 3D and 4D HSQC-NOESY-TROSY experiments are enhanced by 62% and 8% at 5 °C, respectively, compared to their corresponding 3D and 4D HSQC-NOESY-HSQC experiments. Furthermore, the corresponding linewidths are, on average, decreased by 20% and 18% Hz in the HN and N2 dimensions, respectively. This enhancement of sensitivity depends on the molecular mass of the sample used and the lengths of the evolution times in the indirectly and directly detected dimensions.
Similar content being viewed by others
References
Delaglio, F., Grzesiek, S., Vuister, G., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR, 6, 277–293.
Frenkiel, T., Bauer, C., Carr, M.C., Birdsall, B. and Feeney, J. (1990) J. Magn. Reson., 90, 420–425.
Ikura, M., Bax, A., Clore, G.M. and Gronenborn, A.M. (1990) J. Am. Chem. Soc., 112, 9020–9022.
Kay, L.E., Clore, G.M., Bax, A. and Gronenborn, A.M. (1990) Science, 249, 411–414.
Kay, L.E., Keifer, P. and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663–10665.
Meissner, A., Duus, O.J. and Sørensen, O.W. (1997) J. Biomol. NMR, 10, 89–94.
Muhandiram, D.R., Xu, G.Y. and Kay, L.E. (1993) J. Biomol. NMR, 3, 463–470.
Palmer, A.G., Cavanagh, J., Wright, P.E. and Rance, M. (1991) J. Magn. Reson., 93, 151–170.
Pervushin, K., Riek, R., Wider, G. and Wüthrich, K. (1997) Proc. Natl. Acad. Sci. USA, 94, 12366–12371.
Pervushin, K., Riek, R., Wider, G. and Wüthrich, K. (1998) J. Am. Chem. Soc., 120, 6394–6400.
Pervushin, K., Wider, G., Riek, R. and Wüthrich, K. (1999) Proc. Natl. Acad. Sci. USA, 96, 9607–9612.
Salzmann, M., Pervushin, K., Wider, G., Senn, H. and Wüthrich, K. (1998) Proc. Natl. Acad. Sci. USA, 95, 13585–13590.
Salzmann, M., Wider, G., Pervushin, K., Senn, H. and Wüthrich, K. (1999a) J. Am. Chem. Soc., 121, 844–848.
Salzmann, M., Pervushin, K., Wider, G., Senn, H. and Wüthrich, K. (1999b) J. Biomol. NMR, 14, 85–88.
Sørensen, M.D., Meissner, A. and Sørensen, O.W. (1997) J. Biomol. NMR, 10, 181–186.
Torchia, D.A., Sparks, S.W. and Bax, A. (1988) J. Am. Chem. Soc., 110, 2320–2321.
Venters, R.A., Metzler, W.J., Spicer, L.D., Mueller, L. and Farmer II, B.T. (1995) J. Am. Chem. Soc., 117, 9592–9595.
Vuister, G.W., Clore, G.M., Gronenborn, A.M., Powers, R., Garrett, D.S., Tschudin, R. and Bax, A. (1993) J. Magn. Reson., B101, 210–213.
Weigelt, J. (1998) J. Am. Chem. Soc., 120, 10778–10779.
Yang, D. and Kay, L.E. (1999) J. Biomol. NMR, 13, 3–10.
Zhu, G., Kong, X.M., Yan. X.Z. and Sze, K.H. (1998) Angew. Chem. Int. Ed. Engl., 37, 2859–2861.
Zhu, G., Kong, X.M. and Sze, K.H. (1999a) J. Biomol. NMR, 13, 77–81.
Zhu, G., Xia, Y., Sze, K.H. and Yan, X. (1999b) J. Biomol. NMR, 14, 377–381.
Zuiderweg, E.R.P., Petros, A.M., Fesik, S.W. and Olejniczak, E.T. (1991) J. Am. Chem. Soc., 113, 370–372.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xia, Y., Sze, K. & Zhu, G. Transverse relaxation optimized 3D and 4D 15N/15N separated NOESY experiments of 15N labeled proteins. J Biomol NMR 18, 261–268 (2000). https://doi.org/10.1023/A:1026590201757
Issue Date:
DOI: https://doi.org/10.1023/A:1026590201757