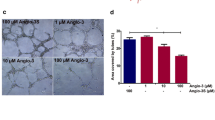
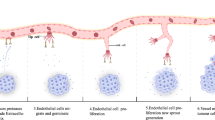
Abstract
Angiogenesis has been acknowledged as an important requirement for growth and metastasis of tumors. Complete or partial suppression of vascular growth by a number of different strategies has been consistently associated with suppression of tumor expansion and even reduction of tumor burden. Consequently, identification of the molecular pathways of the angiogenic response has been a major focus of interest in academia and industry. The development of tumor-specific anti-angiogenic therapy was also catalyzed by the finding that inhibitors of angiogenesis appeared immune to the development of drug resistance by the tumor cells, a major restrain in current chemotherapy. Although the full identification of players and their cross-talk is still at its infancy, it appears that partial blockade of one of the steps in the angiogenesis cascade, is sufficient to affect capillary morphogenesis. Thus, suppression of specific integrin pathways or vascular endothelial growth factor signaling have been shown effective in the suppression of tumor-mediated angiogenesis and have led to subsequent initiation of clinical trials.
In addition to the generation of antibodies or chemical mimetics to interfere with particular steps during vascular organization, several endogenous (or physiological) molecules have also been identified. The list of endogenous modulators of angiogenesis is growing and can offer additional and important tool for the generation of therapies to restrain tumor vascularization. This review will focus one group of such molecules which include the thrombospondins and metallospondins, two families of proteins linked by the presence of a conserved anti-angiogenic functional domain.
Similar content being viewed by others
References
Hanahan D, Weinberg RA: The hallmarks of cancer. Cell 100(1): 57-70, 2000
Takayama K, Ueno H, Nakanishi Y, Sakamoto T, Inoue K, Shimizu K, Oohashi H, Hara N: Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res 60(8): 2169-77, 2000
Holash J, Wiegand SJ, Yancopoulos GD: New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 18(38): 5356-62, 1999
Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G: Neuropilin-2 and neuropilin-1 are receptors for the 165-amino acid form of vascular endothelial growth factor (VEGF) and of placenta growth factor-2, but only neuropilin-2 functions as a receptor for the 145-amino acid form of VEGF. J Biol Chem 275(24): 18 040-5, 2000
Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, Klagsbrun M: Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: in vivo expression and antitumor activity. Proc Natl Acad Sci USA 97(6): 2573-8, 2000
O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J: Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88(2): 277-85, 1997
O'Reilly MS, Holmgren L, Chen C, Folkman J: Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 2(6): 689-92, 1996
O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J: Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79(2): 315-28, 1994
Clapp C, Martial JA, Guzman RC, Rentier-Delure F, Weiner RI: The 16-kilodalton N-terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Endocrinology 133(3): 1292-9, 1993
Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N: Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med 6(1): 41-8, 2000
Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M: Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci USA 96(26): 14 888-93, 1999
Jouan V, Canron X, Alemany M, Caen JP, Quentin G, Plouet J, Bikfalvi A: Inhibition of in vitro angiogenesis by platelet factor-4-derived peptides and mechanism of action. Blood 94(3): 984-93, 1999
Vázquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML: METH-1, a human ortholog of ADAMTS-1, and METH-1 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem 274: 23 349-57, 1999
Bornstein P, Sage EH: Thrombospondins. In: Ruoslahti E, Engvall E (eds) Methods in Enzymology, Vol 245, Academic Press, San Diego, CA, 1994, pp 62-85
Bornstein P: Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 130: 503-6, 1995
Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N: Peptides derived from two separate domains of the matrix protein thrombospondin-1 have antiangiogenic activity. J Cell Biol. 122(2): 497-511, 1993
Guo NH, Krutzsch HC, Inman JK, Shannon CS, Roberts DD: Antiproliferative and antitumor activities of Dreverse peptides derived from the second type-1 repeat of Thrombospondin-1. Pept Res 57: 1735-42, 1997
Iruela-Arispe ML, Lombardo M, Krutzsch H, Lawler J, Roberts DD: Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation 100: 1423-31, 1999
Vogel T, Guo NH, Krutzsch HC, Blake DA, Hartman J, Mendelovitz S, Panet A, Roberts DD: Modulation of endothelial cell proliferation, adhesion, and motility by recombinant heparin-binding domain and synthetic peptides from the type I repeats of thrombospondin. J Cell Biochem 53: 74-84, 1993
Taraboletti G, Belotti D, Borsotti P, Vergani V, Rusnati M, Presta M, Giavazzi R: The 140-kilodalton antiangiogenic fragment of thrombospondin-1 binds to basic fibroblast growth factor. Cell Growth Differ 8: 471-9, 1997
Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP: CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol 138: 707-17, 1997
Greenwalt DE, Lipsky RH, Ockenhouse CF, Ikeda H, Tandon NN, Jamieson GA: Membrane glycoprotein CD36: a review of its role in adherence, signal transduction, and transfusion medicine. Blood 80: 1105-15, 1992
Jiménez B, Volpert OV, Crawford SE, Bebbraio M, Silverstein RL, Bouck NP: Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med 6: 41-8, 2000
Murphy-Ulrich JE, Schultz-Cherry S, Höök M: Transforming growth factor-β complexes with thrombospondin. Mol Biol Cell 3: 181-8, 1992
Crawford SE, Stellmach V, Murphy-Ulrich JE, Ribeiro MF, Lawler J, Hynes RO, Boivin GP, Bouck N: Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell 93: 1159-70, 1998
Pepper MS: Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev 8: 21-43, 1997
Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA: Integrin alphaV beta3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 7: 1157-64, 1994
Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn GH, Hynes RO: Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 101: 982-92, 1998
Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Borustein P: Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol 140: 419-30, 1998
Zabrenetzky V, Harris CC, Steeg PS, Roberts DD: Expression of the extracellular matrix molecule thrombospondin inversely correlates with malignant progression in melanoma, lung and breast carcinoma cell lines. Int J Cancer 59: 191-5, 1994
Campbell SC, Volpert OV, Ivanovich M, Bouck NP: Molecular mediators of angiogenesis in bladder cancer. Cancer Res 58: 1298-304, 1998
Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K, Frazier WA, Roberts DD, Steeg PS: Transfection of thrombospondin-1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res 54: 6504-11, 1994
Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M: Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol 155: 441-52, 1999
Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M: Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci USA 96: 14 888-93, 1999
Bleuel K, Popp S, Fusenig NE, Stanbridge EJ, Boukamp P: Tumor suppression in human skin carcinoma cells by chromosome 15 transfer or thrombospondin-1 overexpression through halted tumor vascularization. Proc Natl Acad Sci USA 96: 2065-70, 1999
Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K: Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem 272: 556-62, 1997
Kuno K, Matsushima K: ADAMTS-1 protein anchors at the extracellular matrix through the thrombospondin type I motifs and its spacing region. J Biol Chem 273: 13 912-17, 1998
Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, Imai T, Wang Y, Ogata M, Nishimatsu H, Moriyama N, Oh-hashi Y, Morita H, Ishikawa T, Nagai R, Yazaki Y, Matsushima K: ADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest 105: 1345-52, 2000
Espey LL, Yoshioka S, Russell DL, Robker RL, Fujii S, Richards JS: Ovarian expression of a disintegrin and metalloproteinase with thrombospondin motifs during ovulation in the gonadotropin-primed immature rat. Biol Reprod 62: 1090-5, 2000
Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS: Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA 97: 4689-94, 2000
Colige A, Li SW, Sieron AL, Nusgens BV, Prockop DJ, Lapiere CM: cDNA cloning and expression of bovine procollagen I N-proteinase: a new member of the superfamily of zinc-metalloproteinases with binding sites for cells and other matrix components. Proc Natl Acad Sci USA 94: 2374-9, 1997
Tang BL, Hong W: ADAMTS: a novel family of proteases with an ADAM protease domain and thrombospondin 1 repeats. FEBS Lett 445: 223-5, 1999
Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Decicco CP, Wynn R, Rockwell A, Yang F, Duke JL, Solomon K, George H, Bruckner R, Nagase H, Itoh Y, Ellis DM, Ross H, Wiswall BH, Murphy K, Hillman MC Jr, Hollis GF, Arner EC et al.: Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science 284: 1664-6, 1999
Hurskainen TL, Hirohata S, Seldin MF, Apte SS: ADAMTS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem 274: 25 555-63, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carpizo, D., Iruela-Arispe, M.L. Endogenous Regulators of Angiogenesis – Emphasis on Proteins with Thrombospondin – Type I Motifs. Cancer Metastasis Rev 19, 159–165 (2000). https://doi.org/10.1023/A:1026570331022
Issue Date:
DOI: https://doi.org/10.1023/A:1026570331022