Abstract
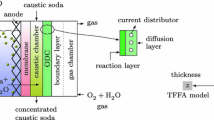
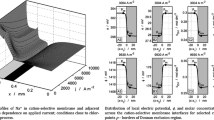
A theoretical study of current density and potential at the anode, membrane and cathode, of a chlor-alkali membrane cell where the electrode blades are placed vertically, is presented. A representative unit cell is modelled in primary, secondary and pseudo-tertiary current distribution models. It is shown that electrolyte and membrane resistance has the greatest effect on current distribution. Furthermore, it is shown that there is a surprisingly small influence of mass transport on current distribution, on the assumption that the diffusion layer is of constant thickness. In converse to this, it is shown that mass transport affects the anode overpotential distribution to the extent that conclusions can be made about the occurrence of side-reactions and where they occur. Finally, it is shown that it is possible to estimate tertiary behaviour with a secondary current distribution model, by using an analytic expression at the anode surface.
Similar content being viewed by others
References
T. Borucinski and K. Schneiders, 'A new generation of the Krupp Uhde single-element design', Mod. Chlor-Alkali Technol. Vol. 7 (Ellis Horwood, Chichester, UK, 1998) 105-112.
A.D. Martin, A.A. Wragg and J.C.R. Turner, 'Numerical mod elling of membrane cell primary current and potential distributions. Part 1: Finite difference approach and effect of electrode shape', IChemE Symposium Series No. 98, (1986), p. 35.
K. Borucinski and K. Schneiders, op. cit. [1], chapter 13. (Ellis Horwood, 1996).
G. Bergner, 'Operating experience gained with the bipolar Hoescht-Udhe membrane cell', Mod. Chlor-Alkali Technol. Vol. 3, (Ellis Horwood, Chichester, UK, 1986), chapter 13.
A.D. Martin and A.A. Wragg, 'Numerical modelling of membrane cell primary current and potential distributions. Part 2: Membrane position, electrode position and electrolyte effects', 4th European symposium on 'Electrochemical Engineering', Prague, Czech Republic (1996).
C. Traini and G. Meneghini, 'Improvement of electrode performance from combined optimization of coating composition and structural design', Mod. Chlor-Alkali Technol. 5 (1992) 269-280.
J.T. Keating, 'sulphate deposition and current distribution in membranes for chlor-alkali cells', Proceedings from the symposium on 'Electrochemical Engineering in the Chlor-Alkali and Chlorate Industries', Electrochemical Society, (1988).
J.S. Newman, 'Electrochemical Systems', 2nd edn (Prentice-Hall, Englewood Cliffs, NJ, 1991).
H. Vogt, 'Comprehensive Treatise of Electrochemistry', Vol. 6 (1983a), p. 445.
N. Ibl and J. Venczel, Met. Oderfläche 24 (1970) 1105.
J. Bard, 'Encyclopaedia of Electrochemistry of the Elements', Vol. 1 (Marcel Dekker, New York, 1973).
S. Rondinini and M. Ferrari, 'Resistivity behaviour of perfluori-nated ionic membranes in the chlor-alkali electrolytic process', Proceedings Electrochemical Society (1986), volume 86-13.
N. Ibl, E. Adams, J. Venczel and E. Schalch, Chem. Ing. Tech. 43 (1971) 202-215.
D. Simonsson and G. Lindbergh, 'Elektrokemi och korrosion', Applied Electrochemistry, Royal Institute of Technology, Stockholm (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Byrne, P., Bosander, P., Parhammar, O. et al. A primary, secondary and pseudo-tertiary mathematical model of a chlor-alkali membrane cell. Journal of Applied Electrochemistry 30, 1361–1367 (2000). https://doi.org/10.1023/A:1026530830265
Issue Date:
DOI: https://doi.org/10.1023/A:1026530830265