Abstract
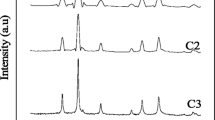
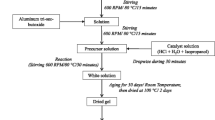
The structure of iron oxide was controlled by regulating the hydrolytic polymerization of aquo iron complexes with organic polydentate ligands such as diols. Iron oxides were prepared by calcining the precursor polymers obtained from iron nitrate nonahydrate and diols. When the diols were 1,2-pentanediol, 1,2-hexanediol and 1,2-octanediol, α -Fe2O3 with corundum structure appeared exclusively or as the main crystalline phase, in spite of the amount of diol used and the calcination temperature. In the case of 1,2-decanediol and 1,2-dodecanediol, when five moles of the diols were used to one mole of iron nitrate and the calcination temperatures were below 400° C, γ -Fe2O3 with spinel structure appeared as the main phase and, when less than five moles of the diols were used, α -Fe2O3 appeared exclusively or as the main phase, irrespective of the calcination temperature. This tendency was also observed in thin films. Thus, a transparent magnetic film composed of γ -Fe2O3 could be prepared by applying a benzene solution of the iron polymer, obtained with 5 equivalents of 1,2-decanediol, on quartz and calcining the gel film at 350° C.
Similar content being viewed by others
References
R.B. Heslop and P.L. Robinson, Inorganic Chemistry (Elsevier, Maruzen Asian Edition, Japan, 1960), p. 465.
C.M. Flynn, Jr., Chem. Rev. 84, 31 (1984).
F. Basolo and R.G. Pearson, Mechanisms of Inorganic Reactions, A Study of Metal Complexes in Solution (Wiley, NewYork, 1968).
K. Wieghardt, K. Phol, I. Jibril, and G. Huttner, Angew. Chem. Int. Ed. Engl. 23, 77 (1984).
S.M. Gorun and S.J. Lippard, Nature 319, 666 (1986).
S.J. Lippard, Chem. Br., 222 (1986).
W.H. Armstrong, M.E. Roth, and S.J. Lippard, J. Am. Chem. Soc. 109, 6318 (1987).
F. Mizukami, Y. Kobayashi, S. Niwa, M. Toba, and K. Shimizu, J. Chem. Soc. Chem. Commun., 1540 (1988).
F. Mizukami, M. Fujii, S. Niwa, and M. Toba, J. Sol-Gel Sci. Tech. 2, 359 (1994).
K. Kojima, F. Mizukami, M. Miyazaki, and K. Maeda, J. NonCryst. Solids 147&148, 442 (1992).
K. Maeda, F. Mizukami, S. Miyashita, S. Niwa, and M. Toba, J. Chem. Soc. Chem. Commun., 1268 (1990).
T. Nishide and F. Mizukami, Thin Solid Films 259, 212 (1995).
Rights and permissions
About this article
Cite this article
Kojima, K., Miyazaki, M., Mizukami, F. et al. Selective Formation of Spinel Iron Oxide in Thin Films by Complexing Agent-Assisted Sol-Gel Processing. Journal of Sol-Gel Science and Technology 8, 77–81 (1997). https://doi.org/10.1023/A:1026407306265
Issue Date:
DOI: https://doi.org/10.1023/A:1026407306265